Effectiveness of the Original Monovalent Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccination Series Against Hospitalization for COVID-19-Associated Venous Thromboembolism
- PMID: 39405261
- PMCID: PMC12063076
- DOI: 10.1093/infdis/jiae502
Effectiveness of the Original Monovalent Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccination Series Against Hospitalization for COVID-19-Associated Venous Thromboembolism
Abstract
Background: Coronavirus disease 2019 (COVID-19) is a strong risk factor for venous thromboembolism (VTE). Few studies have evaluated the effectiveness of COVID-19 vaccination in preventing hospitalization for COVID-19 with VTE.
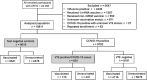
Methods: Adults hospitalized at 21 sites between March 2021 and October 2022 with symptoms of acute respiratory illness were assessed for COVID-19, completion of the original monovalent messenger RNA (mRNA) COVID-19 vaccination series, and VTE. Prevalence of VTE was compared between unvaccinated and vaccinated patients with COVID-19. The vaccine effectiveness (VE) in preventing COVID-19 hospitalization with VTE was calculated using a test-negative design. The VE was also stratified by predominant circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant.
Results: Among 18 811 patients (median age [interquartile range], 63 [50-73] years; 49% women; 59% non-Hispanic white, 20% non-Hispanic black, and 14% Hispanic; and median of 2 comorbid conditions [interquartile range, 1-3]), 9792 were admitted with COVID-19 (44% vaccinated), and 9019 were test-negative controls (73% vaccinated). Among patients with COVID-19, 601 had VTE diagnosed by hospital day 28, of whom 170 were vaccinated. VTE was more common among unvaccinated than vaccinated patients with COVID-19 (7.8% vs 4.0%; P = .001). The VE against COVID-19 hospitalization with VTE was 84% overall (95% confidence interval, 80%-87%), and VE stratified by predominant circulating variant was 88% (73%-95%) for Alpha, 93% (90%-95%) for Delta, and 68% (58%-76%) for Omicron variants.
Conclusions: Vaccination with the original monovalent mRNA series was associated with a decrease in COVID-19 hospitalization with VTE, though data detailing prior history of VTE and use of anticoagulation were not available. These findings will inform risk-benefit considerations for those considering vaccination.
Keywords: COVID-19; SARS-CoV-2; vaccine; venous thromboembolism.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . M. B. S. reports consulting fees from Bristol Myers Squibb, Janssen, Pfizer, CSL Behrin, and Attralus and honoraria from Bristol Myers Squibb, Janssen, and Pfizer, and he has participated on a data and safety monitoring board on behalf of CSL Behring. I. D. P. reports support from Janssen Pharmaceutical and institutional support from Bluejay Diagnostics and Regeneron. A. S. L. reports support from FluLab and Roche Pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Heit JA, Melton LJ III, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc 2001; 76:1102–10. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

