Dramatic changes in mitochondrial subcellular location and morphology accompany activation of the CO2 concentrating mechanism
- PMID: 39405346
- PMCID: PMC11513932
- DOI: 10.1073/pnas.2407548121
Dramatic changes in mitochondrial subcellular location and morphology accompany activation of the CO2 concentrating mechanism
Abstract
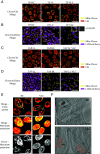
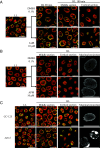
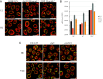
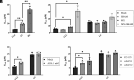
Dynamic changes in intracellular ultrastructure can be critical for the ability of organisms to acclimate to environmental conditions. Microalgae, which are responsible for ~50% of global photosynthesis, compartmentalize their Ribulose 1,5 Bisphosphate Carboxylase/Oxygenase (Rubisco) into a specialized structure known as the pyrenoid when the cells experience limiting CO2 conditions; this compartmentalization is a component of the CO2 Concentrating Mechanism (CCM), which facilitates photosynthetic CO2 fixation as environmental levels of inorganic carbon (Ci) decline. Changes in the spatial distribution of mitochondria in green algae have also been observed under CO2 limitation, although a role for this reorganization in CCM function remains unclear. We used the green microalga Chlamydomonas reinhardtii to monitor changes in mitochondrial position and ultrastructure as cells transition between high CO2 and Low/Very Low CO2 (LC/VLC). Upon transferring cells to VLC, the mitochondria move from a central to a peripheral cell location and orient in parallel tubular arrays that extend along the cell's apico-basal axis. We show that these ultrastructural changes correlate with CCM induction and are regulated by the CCM master regulator CIA5. The apico-basal orientation of the mitochondrial membranes, but not the movement of the mitochondrion to the cell periphery, is dependent on microtubules and the MIRO1 protein, with the latter involved in membrane-microtubule interactions. Furthermore, blocking mitochondrial respiration in VLC-acclimated cells reduces the affinity of the cells for Ci. Overall, our results suggest that mitochondrial repositioning functions in integrating cellular architecture and energetics with CCM activities and invite further exploration of how intracellular architecture can impact fitness under dynamic environmental conditions.
Keywords: CO2 concentrating mechanism; Chlamydomonas; fluorescence microscopy; microalgae; mitochondria.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Update of
-
Dramatic Changes in Mitochondrial Subcellular Location and Morphology Accompany Activation of the CO2 Concentrating Mechanism.bioRxiv [Preprint]. 2024 Mar 27:2024.03.25.586705. doi: 10.1101/2024.03.25.586705. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2407548121. doi: 10.1073/pnas.2407548121. PMID: 38585955 Free PMC article. Updated. Preprint.
Similar articles
-
Dramatic Changes in Mitochondrial Subcellular Location and Morphology Accompany Activation of the CO2 Concentrating Mechanism.bioRxiv [Preprint]. 2024 Mar 27:2024.03.25.586705. doi: 10.1101/2024.03.25.586705. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2407548121. doi: 10.1073/pnas.2407548121. PMID: 38585955 Free PMC article. Updated. Preprint.
-
Mitochondrial carbonic anhydrases are needed for optimal photosynthesis at low CO2 levels in Chlamydomonas.Plant Physiol. 2021 Nov 3;187(3):1387-1398. doi: 10.1093/plphys/kiab351. Plant Physiol. 2021. PMID: 34618049 Free PMC article.
-
CO2-dependent migration and relocation of LCIB, a pyrenoid-peripheral protein in Chlamydomonas reinhardtii.Plant Physiol. 2022 Feb 4;188(2):1081-1094. doi: 10.1093/plphys/kiab528. Plant Physiol. 2022. PMID: 34791500 Free PMC article.
-
The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2 : how Chlamydomonas works against the gradient.Plant J. 2015 May;82(3):429-448. doi: 10.1111/tpj.12829. Plant J. 2015. PMID: 25765072 Review.
-
Carbon-concentrating mechanism in a green alga, Chlamydomonas reinhardtii, revealed by transcriptome analyses.J Basic Microbiol. 2009 Feb;49(1):42-51. doi: 10.1002/jobm.200800352. J Basic Microbiol. 2009. PMID: 19253331 Review.
Cited by
-
Compartmentalized Sesquiterpenoid Biosynthesis and Functionalization in the Chlamydomonas reinhardtii Plastid.Chembiochem. 2025 Feb 3;26(5):e202400902. doi: 10.1002/cbic.202400902. Epub 2024 Dec 5. Chembiochem. 2025. PMID: 39589357 Free PMC article.
-
Light-dark dependent changes in chloroplast and mitochondrial activity in Chlamydomonas reinhardtii.Front Plant Sci. 2025 Jul 17;16:1622214. doi: 10.3389/fpls.2025.1622214. eCollection 2025. Front Plant Sci. 2025. PMID: 40747523 Free PMC article.
-
Mitochondrial support of high rates of photosynthesis.Plant Commun. 2025 Mar 10;6(3):101285. doi: 10.1016/j.xplc.2025.101285. Epub 2025 Feb 15. Plant Commun. 2025. PMID: 39955615 Free PMC article. No abstract available.
-
Photosynthetic Electron Flows and Networks of Metabolite Trafficking to Sustain Metabolism in Photosynthetic Systems.Plants (Basel). 2024 Oct 28;13(21):3015. doi: 10.3390/plants13213015. Plants (Basel). 2024. PMID: 39519934 Free PMC article. Review.
References
-
- van Bergeijk P., Hoogenraad C. C., Kapitein L. C., Right time, right place: Probing the functions of organelle positioning. Trends Cell Biol. 26, 121–134 (2016). - PubMed
-
- Klecker T., Scholz D., Förtsch J., Westermann B., The yeast cell cortical protein NUM1 integrates mitochondrial dynamics into cellular architecture. J. Cell Sci. 126, 2924–2930 (2013). - PubMed
-
- Verstreken P., et al. , Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365–378 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

