A master regulator of central carbon metabolism directly activates virulence gene expression in attaching and effacing pathogens
- PMID: 39405360
- PMCID: PMC11508082
- DOI: 10.1371/journal.ppat.1012451
A master regulator of central carbon metabolism directly activates virulence gene expression in attaching and effacing pathogens
Abstract
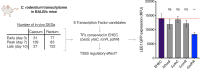
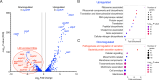
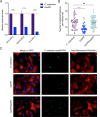
The ability of the attaching and effacing pathogens enterohaemorrhagic Escherichia coli (EHEC) and Citrobacter rodentium to overcome colonisation resistance is reliant on a type 3 secretion system used to intimately attach to the colonic epithelium. This crucial virulence factor is encoded on a pathogenicity island known as the Locus of Enterocyte Effacement (LEE) but its expression is regulated by several core-genome encoded transcription factors. Here, we unveil that the core transcription factor PdhR, traditionally known as a regulator of central metabolism in response to cellular pyruvate levels, is a key activator of the LEE. Through genetic and molecular analyses, we demonstrate that PdhR directly binds to a specific motif within the LEE master regulatory region, thus activating type 3 secretion directly and enhancing host cell adhesion. Deletion of pdhR in EHEC significantly impacted the transcription of hundreds of genes, with pathogenesis and protein secretion emerging as the most affected functional categories. Furthermore, in vivo studies using C. rodentium, a murine model for EHEC infection, revealed that PdhR is essential for effective host colonization and maximal LEE expression within the host. Our findings provide new insights into the complex regulatory networks governing bacterial pathogenesis. This research highlights the intricate relationship between virulence and metabolic processes in attaching and effacing pathogens, demonstrating how core transcriptional regulators can be co-opted to control virulence factor expression in tandem with the cell's essential metabolic circuitry.
Copyright: © 2024 Wale et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

