Thermal Decomposition of 2-Cyclopentenone
- PMID: 39405375
- PMCID: PMC11514027
- DOI: 10.1021/acs.jpca.4c05532
Thermal Decomposition of 2-Cyclopentenone
Abstract
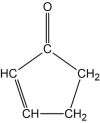
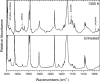
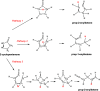
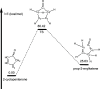
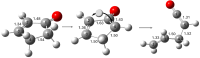
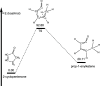
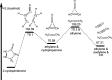
The thermal decomposition of 2-cyclopentenone, a cyclic oxygenated hydrocarbon that occurs in the pyrolysis of biomass, has been studied in a combined experimental and theoretical approach. Gas-phase pyrolysis was performed at temperatures ranging from 1000 to 1400 K in a pulsed, microtubular reactor. Products were identified by FTIR spectroscopy following their isolation in a low-temperature argon matrix. The following products were identified: carbon monoxide, ketene, propenylketene, vinylacetylene, ethylene, propene, acrolein, acetylene, propyne, and propargyl radical. Computational results identify three different decomposition channels involving a H atom migration, and producing prop-2-enylketene (Pathway 1), prop-1-enylketene (Pathway 2), and a second conformation of prop-2-enylketene (Pathway 3). A fourth decomposition pathway involves simultaneous rupture of two C-C bonds forming a high energy cyclopropenone intermediate that further reacts to form ethylene, acetylene, and carbon monoxide. Finally, a fifth pathway to the formation of acrolein and acetylene was identified that proceeds via a multistep mechanism, and an interconversion from 2-cyclopentenone to 3-cyclopentenone was identified computationally, but not observed experimentally.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Anca-Couce A. Reaction Mechanisms and Multi-Scale Modelling of Lignocellulosic Biomass Pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. 10.1016/j.pecs.2015.10.002. - DOI
-
- Vamvuka D. Bio-Oil, Solid and Gaseous Biofuels from Biomass Pyrolysis Processes—an Overview. Int. J. Energy Res. 2011, 35, 835–862. 10.1002/er.1804. - DOI
-
- Nachenius R. W.; Ronsse F.; Venderbosch R. H.; Prins W. Biomass Pyrolysis. Adv. Chem. Eng. 2013, 42, 75–139. 10.1016/B978-0-12-386505-2.00002-X. - DOI
-
- Charon N.; Ponthus J.; Espinat D.; Broust F.; Volle G.; Valette J.; Meier D. Multi-Technique Characterization of Fast Pyrolysis Oils. J. Anal. Appl. Pyrolysis 2015, 116, 18–26. 10.1016/j.jaap.2015.10.012. - DOI
LinkOut - more resources
Full Text Sources

