HJURP inhibits sensitivity to ferroptosis inducers in prostate cancer cells by enhancing the peroxidase activity of PRDX1
- PMID: 39405980
- PMCID: PMC11525750
- DOI: 10.1016/j.redox.2024.103392
HJURP inhibits sensitivity to ferroptosis inducers in prostate cancer cells by enhancing the peroxidase activity of PRDX1
Abstract
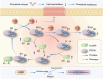
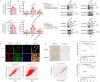
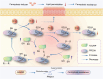
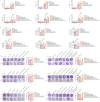
Ferroptosis induction has emerged as a promising therapeutic approach for prostate cancer (PCa), either as a monotherapy or in combination with hormone therapy. Therefore, identifying the mechanisms regulating ferroptosis in PCa cells is essential. Our previous study demonstrated that HJURP, an oncogene upregulated in PCa cells, plays a role in tumor proliferation. Here, we expand these findings by elucidating a novel mechanism by which HJURP inhibits sensitivity to ferroptosis inducers in PCa cells via the PRDX1/reactive oxygen species (ROS) pathway in vitro and in vivo. Mechanistically, HJURP forms disulfide-linked intermediates with PRDX1 through Cys327 and Cys457 residues. This disulfide binding promotes PRDX1 redox cycling and inhibits its hyperoxidation. As a result, HJURP enhances the peroxidase activity of PRDX1, leading to a decrease in ROS levels and subsequently suppressing lipid peroxidation induced by ferroptosis inducers. These findings reveal the potential of HJURP/PRDX1 as novel therapeutic targets and biomarkers of ferroptosis in PCa patients.
Keywords: Disulfide binding; Ferroptosis; HJURP; Peroxidase activity; Prostate cancer.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures












References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. - PubMed
-
- Takahashi T. Prostate cancer screening and the golden rule of humanity. BMJ. 2023;381:1390. - PubMed
-
- Preisser F., Abrams-Pompe R.S., Stelwagen P.J., Böhmer D., Zattoni F., Magli A., et al. European association of urology biochemical recurrence risk classification as a decision tool for salvage radiotherapy-a multicenter study. Eur. Urol. 2023;85:164–170. - PubMed
-
- Sandhu S., Moore C.M., Chiong E., Beltran H., Bristow R.G., Williams S.G. Prostate cancer. Lancet. 2021;398:1075–1090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

