Methionine oxidation of actin cytoskeleton attenuates traumatic memory retention via reactivating dendritic spine morphogenesis
- PMID: 39405981
- PMCID: PMC11525628
- DOI: 10.1016/j.redox.2024.103391
Methionine oxidation of actin cytoskeleton attenuates traumatic memory retention via reactivating dendritic spine morphogenesis
Abstract
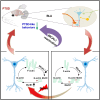
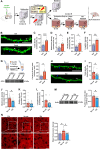
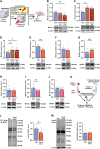
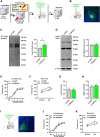
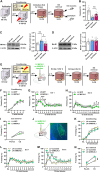
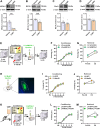
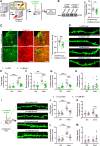
Post-traumatic stress disorder (PTSD) is characterized by hypermnesia of the trauma and a persistent fear response. The molecular mechanisms underlying the retention of traumatic memories remain largely unknown, which hinders the development of more effective treatments. Utilizing auditory fear conditioning, we demonstrate that a redox-dependent dynamic pathway for dendritic spine morphogenesis in the basolateral amygdala (BLA) is crucial for traumatic memory retention. Exposure to a fear-induced event markedly increased the reduction of oxidized filamentous actin (F-actin) and decreased the expression of the molecule interacting with CasL 1 (MICAL1), a methionine-oxidizing enzyme that directly oxidizes and depolymerizes F-actin, leading to cytoskeletal dynamic abnormalities in the BLA, which impairs dendritic spine morphogenesis and contributes to the persistence of fearful memories. Following fear conditioning, overexpression of MICAL1 in the BLA inhibited freezing behavior during fear memory retrieval via reactivating cytokinesis, whereas overexpression of methionine sulfoxide reductase B 1, a key enzyme that reduces oxidized F-actin monomer, increased freezing behavior during retrieval. Notably, intra-BLA injection of semaphorin 3A, an endogenous activator of MICAL1, rapidly disrupted fear memory within a short time window after conditioning. Collectively, our results indicate that redox modulation of actin cytoskeleton in the BLA is functionally linked to fear memory retention and PTSD-like memory.
Keywords: Actin cytoskeleton; Basolateral amygdala; Cued fear conditioning; Dendritic spine; Molecule interacting with CasL 1.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

