Synaptic neoteny of human cortical neurons requires species-specific balancing of SRGAP2-SYNGAP1 cross-inhibition
- PMID: 39406239
- PMCID: PMC11546603
- DOI: 10.1016/j.neuron.2024.08.021
Synaptic neoteny of human cortical neurons requires species-specific balancing of SRGAP2-SYNGAP1 cross-inhibition
Abstract
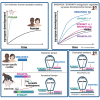
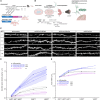
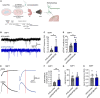
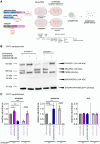
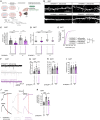
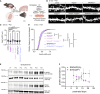
Human-specific (HS) genes have been implicated in brain evolution, but their impact on human neuron development and diseases remains unclear. Here, we study SRGAP2B/C, two HS gene duplications of the ancestral synaptic gene SRGAP2A, in human cortical pyramidal neurons (CPNs) xenotransplanted in the mouse cortex. Downregulation of SRGAP2B/C in human CPNs led to strongly accelerated synaptic development, indicating their requirement for the neoteny that distinguishes human synaptogenesis. SRGAP2B/C genes promoted neoteny by reducing the synaptic levels of SRGAP2A,thereby increasing the postsynaptic accumulation of the SYNGAP1 protein, encoded by a major intellectual disability/autism spectrum disorder (ID/ASD) gene. Combinatorial loss-of-function experiments in vivo revealed that the tempo of synaptogenesis is set by the reciprocal antagonism between SRGAP2A and SYNGAP1, which in human CPNs is tipped toward neoteny by SRGAP2B/C. Thus, HS genes can modify the phenotypic expression of genetic mutations leading to ID/ASD through the regulation of human synaptic neoteny.
Keywords: SRGAP2; SYNGAP1; autism spectrum disorder; cortical neuron; human brain development; intellectual deficiency; neoteny; synapse.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Sherwood C.C., Gómez-Robles A. Brain Plasticity and Human Evolution. Annu. Rev. Anthropol. 2017;46:399–419. doi: 10.1146/annurev-anthro-102215-100009. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

