Cardiomyocyte-specific knockout of ADAM17 alleviates doxorubicin-induced cardiomyopathy via inhibiting TNFα-TRAF3-TAK1-MAPK axis
- PMID: 39406701
- PMCID: PMC11480360
- DOI: 10.1038/s41392-024-01977-z
Cardiomyocyte-specific knockout of ADAM17 alleviates doxorubicin-induced cardiomyopathy via inhibiting TNFα-TRAF3-TAK1-MAPK axis
Abstract
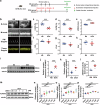
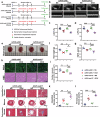
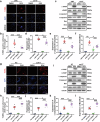
The pathogenesis of doxorubicin-induced cardiomyopathy remains unclear. This study was carried out to test our hypothesis that ADAM17 aggravates cardiomyocyte apoptosis induced by doxorubicin and inhibition of ADAM17 may ameliorate doxorubicin-induced cardiomyopathy. C57BL/6J mice were intraperitoneally injected with a cumulative dose of doxorubicin to induce cardiomyopathy. Cardiomyocyte-specific ADAM17-knockout (A17α-MHCKO) and ADAM17-overexpressing (AAV9-oeA17) mice were generated. In addition, RNA sequencing of the heart tissues in different mouse groups and in vitro experiments in neonatal rat cardiomyocytes (NRCMs) receiving different treatment were performed. Mouse tumor models were constructed in A17fl/fl and A17α-MHCKO mice. In addition, cardiomyocyte-specific TRAF3-knockdown and TRAF3-overexpressing mice were generated. ADAM17 expression and activity were markedly upregulated in doxorubicin-treated mouse hearts and NRCMs. A17α-MHCKO mice showed less cardiomyocyte apoptosis induced by doxorubicin than A17fl/fl mice, and cardiomyocyte ADAM17 deficiency did not affect the anti-tumor effect of doxorubicin. In contrast, AAV9-oeA17 mice exhibited markedly aggravated cardiomyocyte apoptosis relative to AAV9-oeNC mice after doxorubicin treatment. Mechanistically, doxorubicin enhanced the expression of transcription factor C/EBPβ, leading to increased expression and activity of ADAM17 in cardiomyocyte, which enhanced TNF-α shedding and upregulated the expression of TRAF3. Increased TRAF3 promoted TAK1 autophosphorylation, resulting in activated MAPKs pathway and cardiomyocyte apoptosis. ADAM17 acted as a positive regulator of cardiomyocyte apoptosis and cardiac remodeling and dysfunction induced by doxorubicin by upregulating TRAF3/TAK1/MAPKs signaling. Thus, targeting ADAM17/TRAF3/TAK1/MAPKs signaling holds a promising potential for treating doxorubicin-induced cardiotoxicity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- 82000411/National Natural Science Foundation of China (National Science Foundation of China)
- 82030051/National Natural Science Foundation of China (National Science Foundation of China)
- 82241203/National Natural Science Foundation of China (National Science Foundation of China)
- 81920108003/National Natural Science Foundation of China (National Science Foundation of China)
- 81970366/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

