Identification of Shemin pathway genes for tetrapyrrole biosynthesis in bacteriophage sequences from aquatic environments
- PMID: 39406702
- PMCID: PMC11480375
- DOI: 10.1038/s41467-024-52726-3
Identification of Shemin pathway genes for tetrapyrrole biosynthesis in bacteriophage sequences from aquatic environments
Abstract
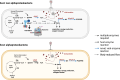
Tetrapyrroles such as heme, chlorophyll, and vitamin B12 are essential for various metabolic pathways. They derive from 5-aminolevulinic acid (5-ALA), which can be synthesized by a single enzyme (5-ALA synthase or AlaS, Shemin pathway) or by a two-enzyme pathway. The genomes of some bacteriophages from aquatic environments carry various tetrapyrrole biosynthesis genes. Here, we analyze available metagenomic datasets and identify alaS homologs (viral alaS, or valaS) in sequences corresponding to marine and freshwater phages. The genes are found individually or as part of complete or truncated three-gene loci encoding heme-catabolizing enzymes. Amino-acid sequence alignments and three-dimensional structure prediction support that the valaS sequences likely encode functional enzymes. Indeed, we demonstrate that is the case for a freshwater phage valaS sequence, as it can complement an Escherichia coli 5-ALA auxotroph, and an E. coli strain overexpressing the gene converts the typical AlaS substrates glycine and succinyl-CoA into 5-ALA. Thus, our work identifies valaS as an auxiliary metabolic gene in phage sequences from aquatic environments, further supporting the importance of tetrapyrrole metabolism in bacteriophage biology.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Shemin, D. & Rittenberg, D. The utilization of glycine for the synthesis of a porphyrin. J. Biol. Chem.159, 567–568 (1945). - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

