Molecular dynamics simulations involving different β-propeller mutations reported in Swiss and French patients correlate with their disease phenotypes
- PMID: 39406775
- PMCID: PMC11480402
- DOI: 10.1038/s41598-024-75070-4
Molecular dynamics simulations involving different β-propeller mutations reported in Swiss and French patients correlate with their disease phenotypes
Abstract
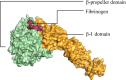
Integrin αIIbβ3 is the predominant receptor for fibrinogen which mediates platelet aggregation, an important step in hemostasis and thrombosis. Several mutations have been reported in the genes encoding αIIb and β3 subunits among patients with Glanzmann thrombasthenia, of which 177 are in the β-propeller domain. The two subunits form a heterodimer at the interface between β-propeller and β-I domains of αIIb and β3, respectively with their stability critical for intracellular trafficking, surface expression, and ligand binding. Our study was aimed at retrieving the β-propeller mutations from various databases and studying structural variations due to select mutations upon interaction with fibrinogen using molecular docking and molecular dynamics. Mutations were studied for their impact on phenotypic severity, structural stability, and evolutionary conservation. Molecular docking analysis and molecular dynamics simulations were carried out for αIIb-β3 complexes as well as αIIbβ3-fibrinogen complexes; in particular, E355K structure had more deviations, fluctuations, and other changes which compromised its structural stability and binding affinity when compared to both wild-type and G401C structures. Our comprehensive in silico analysis clearly reiterates that mutations in the β-propeller are not only responsible for structural changes in this domain but also have implications on the overall structure and function of integrin αIIbβ3.
Keywords: Glanzmann Thrombasthenia; Missense mutations; Molecular docking; Molecular dynamics; Β-propeller; αIIbβ3.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Molecular dynamics simulations corroborate recombinant expression studies carried out on three αIIb β-propeller mutations reported in Indian Glanzmann thrombasthenia patients.J Cell Biochem. 2023 Jul;124(7):989-1001. doi: 10.1002/jcb.30423. Epub 2023 May 21. J Cell Biochem. 2023. PMID: 37210732
-
In silico analysis of structural modifications in and around the integrin αIIb genu caused by ITGA2B variants in human platelets with emphasis on Glanzmann thrombasthenia.Mol Genet Genomic Med. 2018 Mar;6(2):249-260. doi: 10.1002/mgg3.365. Epub 2018 Jan 31. Mol Genet Genomic Med. 2018. PMID: 29385657 Free PMC article.
-
Abnormal cytoplasmic extensions associated with active αIIbβ3 are probably the cause for macrothrombocytopenia in Glanzmann thrombasthenia-like syndrome.Blood Coagul Fibrinolysis. 2015 Apr;26(3):302-8. doi: 10.1097/MBC.0000000000000241. Blood Coagul Fibrinolysis. 2015. PMID: 25806962
-
Glanzmann thrombasthenia: state of the art and future directions.Semin Thromb Hemost. 2013 Sep;39(6):642-55. doi: 10.1055/s-0033-1353393. Epub 2013 Aug 8. Semin Thromb Hemost. 2013. PMID: 23929305 Free PMC article. Review.
-
Are bone defects in rare patients with Glanzmann's thrombasthenia associated with ITGB3 or ITGA2B mutations?Platelets. 2011;22(7):547-51. doi: 10.3109/09537104.2011.573600. Epub 2011 May 11. Platelets. 2011. PMID: 21557682 Review.
References
-
- Xiong, J. P. et al. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an arg-gly-asp ligand. Sci. (1979). 296, 151–155 (2002). - PubMed
-
- Kiyomizu, K. et al. Recognition of highly restricted regions in the β-propeller domain of αIIb by platelet-associated anti-αIIbβ3 autoantibodies in primary immune thrombocytopenia. Blood.120, 1499–1509 (2012). - PubMed
-
- Westrup, D. et al. Glanzmann Thrombasthenia Frankfurt I is associated with a point mutation Thr176IIe in the N-terminal region of αIIb subunit integrin. Thromb. Haemost. 92, 1040–1051 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

