A novel outer membrane vesicle adjuvant improves vaccine protection against Bordetella pertussis
- PMID: 39406780
- PMCID: PMC11480359
- DOI: 10.1038/s41541-024-00990-1
A novel outer membrane vesicle adjuvant improves vaccine protection against Bordetella pertussis
Abstract
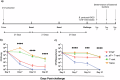
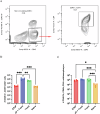
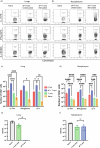
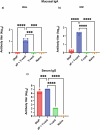
Pertussis is a vaccine-preventable respiratory disease caused by the Gram negative coccobacillus Bordetella pertussis. The licensed acellular pertussis (aP) vaccines protect against disease but do not prevent bacterial colonization and transmission. Here, we developed and tested an intranasal vaccine composed of aP antigens combined with T-vant, a novel adjuvant derived from bacterial outer membrane vesicles, that elicits both mucosal and systemic immune responses. We hypothesized that immunization of mice with aP-T-vant would enhance mucosal immunity and eliminate B. pertussis in the respiratory tract. In contrast to mice immunized intramuscularly with the licensed aP vaccine, intranasal immunization with aP-T-vant eliminated bacteria in both the lung and nasopharynx. Protection was associated with IFN-gamma and IL-17-producing, non-circulating CD4 + T cells in the lung and nasopharynx, and sterilizing immunity in the nasopharynx was dependent on IL-17. Novel mucosal adjuvants, such as T-vant, warrant further investigation to enhance the efficacy of next generation pertussis vaccines.
© 2024. The Author(s).
Conflict of interest statement
J.B.M. and L.A.M. are funded by NIH/NIAID 1R01AI166756-01 and NIH Adjuvant Development Contract 272201800045 C. J.B.M. and L.A.M. are inventors of T-vant and have a patent on the OMV adjuvant platform.
Figures
References
-
- Warfel, J. M. & Merkel, T. J. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol.6, 787–796 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

