Transient CAR T cells with specificity to oncofetal glycosaminoglycans in solid tumors
- PMID: 39406935
- PMCID: PMC11554890
- DOI: 10.1038/s44321-024-00153-8
Transient CAR T cells with specificity to oncofetal glycosaminoglycans in solid tumors
Abstract
Glycosaminoglycans are often deprioritized as targets for synthetic immunotherapy due to the complexity of glyco-epitopes and limited options for obtaining specific subtype binding. Solid tumors express proteoglycans that are modified with oncofetal chondroitin sulfate (CS), a modification normally restricted to the placenta. Here, we report the design and functionality of transient chimeric antigen receptor (CAR) T cells with selectivity to oncofetal CS. Following expression in T cells, the CAR could be "armed" with recombinant VAR2CSA lectins (rVAR2) to target tumor cells expressing oncofetal CS. While unarmed CAR T cells remained inactive in the presence of target cells, VAR2-armed CAR T cells displayed robust activation and the ability to eliminate diverse tumor cell types in vitro. Cytotoxicity of the CAR T cells was proportional to the concentration of rVAR2 available to the CAR, offering a potential molecular handle to finetune CAR T cell activity. In vivo, armed CAR T cells rapidly targeted bladder tumors and increased the survival of tumor-bearing mice. Thus, our work indicates that cancer-restricted glycosaminoglycans may be exploited as potential targets for CAR T cell therapy.
Keywords: CAR T Cells; Chondroitin Sulfate; Immunotherapy; Oncofetal CS; Solid Tumor.
© 2024. The Author(s).
Conflict of interest statement
Figures


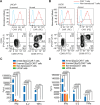
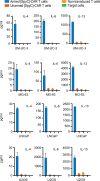
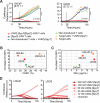

References
-
- Albelda SM (2024) ‘CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn’. Nat Rev Clin Oncol 21:47–66 - PubMed
MeSH terms
Substances
Grants and funding
- 6580/CIHR travel Award- Michael Smith Foreign Study Supplement
- NNF22OC0076055/NNF Distinguished Innovator grant
- NNF21OC0068192/NNF Tandem grant
- P50 CA097186/CA/NCI NIH HHS/United States
- #6456/University of British Columbia (UBC), UBC Four year Fellowships (FYF)
- SU2C-AACR-DT-27-17/St. Baldrick's Foundation/ American Association for Cancer Research/ Stand Up to Cancer Pediatric Dream Team Translational Research Grant
- PJT-153092/Canadian Government | CIHR | Canadian Institutes of Health Research - Antimicrobial Resistance Research Initiative
- 1016339,223493; 5P50 CA097186-17/NIH Prostate Cancer PNW-SPORE
- 01353-000/CIHR-Vanier scholarship
LinkOut - more resources
Full Text Sources

