Activity of polymyxin B combined with cefepime-avibactam against the biofilms of polymyxin B-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae in in vitro and in vivo models
- PMID: 39407114
- PMCID: PMC11481319
- DOI: 10.1186/s12866-024-03571-3
Activity of polymyxin B combined with cefepime-avibactam against the biofilms of polymyxin B-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae in in vitro and in vivo models
Abstract
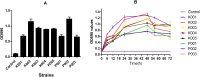
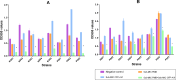
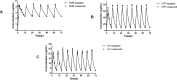
Bacterial biofilms, often forming on medical devices, can lead to treatment failure due to their increased antimicrobial resistance. Cefepime-avibactam (CFP-AVI) exhibits potent activities against Pseudomonas aeruginosa (P. aeruginosa) and Klebsiella pneumoniae (K. pneumoniae) when used with polymyxin B (PMB). However, its efficacy in biofilm-related infections is unknown. The present study aimed to evaluate the activity of PMB combined with CFP-AVI against the biofilms of PMB-resistant Gram-negative bacteria. Five K. pneumoniae strains and three P. aeruginosa strains known to be PMB-resistant and prone to biofilm formation were selected and evaluated. Antimicrobial susceptibility assays demonstrated that the minimal biofilm inhibitory and eradication concentrations of PMB and CFP-AVI for biofilms formed by the eight strains were significantly higher than the minimal inhibitory concentrations of the antibiotics for planktonic cells. The biofilm formation inhibition and eradication assays showed that PMB combined with CFP-AVI cannot only suppress the formation of biofilm but also effectively eradicate the preformed mature biofilms. In a modified in vitro pharmacokinetic/pharmacodynamic biofilm model, CFP-AVI monotherapy exhibited a bacteriostatic or effective activity against the biofilms of seven strains, whereas PMB monotherapy did not have any activity at 72 h. However, PMB combined with CFP-AVI demonstrated bactericidal activity against the biofilms of all strains at 72 h. In an in vivo Galleria mellonella infection model, the 7-day survival rates of larvae infected with biofilm implants of K. pneumoniae or P. aeruginosa were 0-6.7%, 40.0-63.3%, and 46.7-90.0%, respectively, for PMB alone, CFP-AVI alone, and PMB combined with CFP-AVI; the combination therapy increased the rate by 6.7-33.3% (P < 0.05, n = 6), compared to CFP-AVI monotherapy. It is concluded that PMB combined with CFP-AVI exhibits effective anti-biofilm activities against PMB-resistant K. pneumoniae and P. aeruginosa both in vitro and in vivo, and thus may be a promising therapeutic strategy to treat biofilm-related infections.
Keywords: Klebsiella pneumoniae; Pseudomonas aeruginosa; Biofilm; Cefepime-avibactam; Polymyxin B-resistance.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Evaluation of antibiotic efficacy against infections caused by planktonic or biofilm cultures of Pseudomonas aeruginosa and Klebsiella pneumoniae in Galleria mellonella.Int J Antimicrob Agents. 2015 Nov;46(5):538-45. doi: 10.1016/j.ijantimicag.2015.07.014. Epub 2015 Aug 31. Int J Antimicrob Agents. 2015. PMID: 26364845
-
Activity of ceftazidime-avibactam alone and in combination with polymyxin B against carbapenem-resistant Klebsiella pneumoniae in a tandem in vitro time-kill/in vivo Galleria mellonella survival model analysis.Int J Antimicrob Agents. 2020 Jan;55(1):105852. doi: 10.1016/j.ijantimicag.2019.11.009. Epub 2019 Nov 23. Int J Antimicrob Agents. 2020. PMID: 31770627
-
Polymyxin B combined with amikacin delays the resistance of Klebsiella pneumoniae to polymyxin B by modulating the expression of NlpE.Infect Genet Evol. 2025 Sep;133:105780. doi: 10.1016/j.meegid.2025.105780. Epub 2025 Jun 4. Infect Genet Evol. 2025. PMID: 40480594
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
Bacteriophage-derived depolymerase: a review on prospective antibacterial agents to combat Klebsiella pneumoniae.Arch Virol. 2025 Mar 8;170(4):70. doi: 10.1007/s00705-025-06257-x. Arch Virol. 2025. PMID: 40057622 Review.
Cited by
-
Azithromycin represses evolution of ceftazidime/avibactam resistance by translational repression of rpoS in Pseudomonas aeruginosa.J Bacteriol. 2025 May 22;207(5):e0055224. doi: 10.1128/jb.00552-24. Epub 2025 Apr 30. J Bacteriol. 2025. PMID: 40304512 Free PMC article.
References
-
- Walsh TR. Toleman MA.The emergence of pan-resistant gram-negative pathogens merits a rapid global political response[J]. J Antimicrob Chemother. 2012;67(1):1–3. - PubMed
-
- Laxminarayan R, Duse A, Wattal C et al. Antibiotic resistance-the need for global solutions[J]. Lancet Infect Dis. 2013;13(12):1057–98. - PubMed
-
- Vincent JL. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323. - PubMed
-
- Rateb Hina Hedaya,Mcdowell Joan R. S.Minimising central line-associated bloodstream infection rate in inserting central venous catheters in the adult intensive care units[J]. J Clin Nurs. 2017;23-24:26. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

