NIR-II light based combinatorial management of hypertrophic scar by inducing autophagy in fibroblasts
- PMID: 39407227
- PMCID: PMC11481805
- DOI: 10.1186/s12951-024-02876-9
NIR-II light based combinatorial management of hypertrophic scar by inducing autophagy in fibroblasts
Abstract
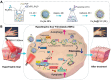
The hypertrophic scar (HS) is a prevalent cutaneous fibrotic disorder that impacts both the aesthetic and functional aspects of the skin, there is an urgent need for a highly safe and effective approach to address the challenge of HS with thick and deep types. Inspired by the superior deep tissue penetrative ability of near-infrared-II (NIR-II) light and potential mitochondria ROS inducing effect of Chinese medicine lycorine (LYC), we fabricated a Cu2Se@LYC (CL) composite by encapsulating LYC on polyvinyl pyrrolidone (PVP) modified Cu2Se nanoparticles. After NIR-II irradiation, CL could induce the generation of reactive oxygen species (ROS) and mitochondrial damage in hypertrophic scar fibroblasts (HSFs). The subsequent release of cytochrome C (cyt-c) from mitochondria into the cytoplasm and upregulation of beclin1 leads to the activation of endogenous apoptosis and autophagy-mediated cell death. The CL + NIR-II treatment exhibited a pronounced anti-scarring effect in both in vitro and in vivo rabbit ear scar models, leading to a significant reduction in the fibrotic markers including Collagen I/III and α-smooth muscle actin (α-SMA). This study comprehensively investigated the crucial role of HSFs' autophagy in scar management and proposed a safe and effective therapy based on NIR-II laser for clinical application.
Keywords: Apoptosis; Autophagy; Hypertrophic scar; Lycorine; Photothermal therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Obaidi N, Keenan C, Chan RK. Burn Scar Management and reconstructive surgery. Surg Clin North Am. 2023;103(3):515–27. - PubMed
-
- Shi N, Wang Y, Xia Z, et al. The regulatory role of the apelin/APJ axis in scarring: identification of upstream and downstream mechanisms. Biochim Biophys Acta Mol Basis Dis. 2024;1870(4):167125. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

