Effectiveness of bivalent HPV vaccination against genital HPV DNA-positivity of a catch-up campaign at age 13-16 years compared to routine vaccination at age 12 years: a biennial repeated cross-sectional study
- PMID: 39407233
- PMCID: PMC11475922
- DOI: 10.1186/s12916-024-03686-4
Effectiveness of bivalent HPV vaccination against genital HPV DNA-positivity of a catch-up campaign at age 13-16 years compared to routine vaccination at age 12 years: a biennial repeated cross-sectional study
Abstract
Background: The Netherlands is one of few countries worldwide which has used the bivalent HPV vaccine for girls-only for over a decade. This allows assessment of vaccine effectiveness (VE) against female genital HPV DNA-positivity of this vaccine in an observational post-licencing real-world setting. Additionally, it is unclear whether catch-up vaccination campaigns result in similar VE as routine vaccination. Therefore, type-specific and grouped VE were assessed and compared for women who had been eligible for catch-up vaccination at 13-16 years with those who had been eligible for routine vaccination at 12 years.
Methods: PASSYON is a Dutch biennial repeated cross-sectional (2011-2021) study among sexual health clinic clients aged 16-24 years old. Women provided self-collected vaginal samples, questionnaires on demographics and sexual behaviour were administered, and women self-reported HPV vaccination status. Samples were analysed using a PCR-based assay (SPF10-LiPA25). Type-specific and grouped VE estimates, adjusted with propensity score stratification, were assessed against genital positivity for 14 HPV types. VE for targeted and non-targeted genotypes were compared between women who had been eligible for the catch-up and those who had been eligible for routine vaccination.
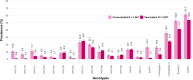
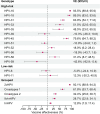
Results: The study included 4488 female participants who had been eligible for HPV vaccination and provided genital swabs (1561 eligible for catch-up, 2927 for routine vaccination). Very high VE against genital HPV-16 and HPV-18 was observed (resp. 93.5% and 89.5%) and significant cross-protection against six other genotypes (HPV-31/33/35/45/52/58), varying from 18.0% (HPV-52) to 79.6% (HPV-45). VE estimates were comparable between women who had been eligible for the catch-up campaign and those eligible for routine vaccination: VE HPV-16/HPV-18: 92.2% (95%CI: 87.9-94.9) vs. 91.8% (95%CI: 86.0-95.2).
Conclusions: In real-world settings, the VE of bivalent vaccine is high against targeted genotypes, with cross-protection against 6 other genotypes. Catch-up campaigns up to age 16 years can be as effective as routine vaccination at age 12, although it is recommendable to provide HPV vaccination at an age at which most are likely not sexually active yet. This may inform countries considering catch-up campaigns when introducing or extending the use of HPV vaccination within their national immunisation programmes.
Keywords: Bivalent vaccine; Catch-up campaign; Human papillomavirus; Observational study; Propensity score; Prophylactic vaccination; Vaccine effectiveness.
© 2024. The Author(s).
Conflict of interest statement
The institution of M.F. Schim van der Loeff and J.C.M Heijne received study funding for an investigator-initiated study from GSK. M.F. Schim van der Loeff served on advisory boards of MSD and Novosanis. All other authors report no conflict of interest.
Figures

References
-
- de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. - PubMed
-
- De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. - PubMed
-
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. - PubMed
-
- Brown DR, Joura EA, Yen GP, Kothari S, Luxembourg A, Saah A, Walia A, Perez G, Khoury H, Badgley D. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine. 2021;39(16):2224–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

