An iron-binding protein of entomopathogenic fungus suppresses the proliferation of host symbiotic bacteria
- PMID: 39407320
- PMCID: PMC11481751
- DOI: 10.1186/s40168-024-01928-4
An iron-binding protein of entomopathogenic fungus suppresses the proliferation of host symbiotic bacteria
Abstract
Background: Entomopathogenic fungal infection-induced dysbiosis of host microbiota offers a window into understanding the complex interactions between pathogenic fungi and host symbionts. Such insights are critical for enhancing the efficacy of mycoinsecticides. However, the utilization of these interactions in pest control remains largely unexplored.
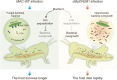
Results: Here, we found that infection by the host-specialist fungus Metarhizium acridum alters the composition of the symbiotic microbiota and increases the dominance of some bacterial symbionts in locusts. Meanwhile, M. acridum also effectively limits the overgrowth of the predominant bacteria. Comparative transcriptomic screening revealed that the fungus upregulates the production of MaCFEM1, an iron-binding protein, in the presence of bacteria. This protein sequesters iron, thereby limiting its availability. Functionally, overexpression of MaCFEM1 in the fungus induces iron deprivation, which significantly suppresses bacterial growth. Conversely, MaCFEM1 knockout relieves the restriction on bacterial iron availability, resulting in iron reallocation. Upon ΔMaCFEM1 infection, some host bacterial symbionts proliferate uncontrollably, turning into opportunistic pathogens and significantly accelerating host death.
Conclusions: This study elucidates the critical role of pathogenic fungal-dominated iron allocation in mediating the shift of host microbes from symbiosis to pathogenicity. It also highlights a unique biocontrol strategy that jointly exploits pathogenic fungi and bacterial symbionts to increase host mortality. Video Abstract.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

