UROPOT: study protocol for a randomized, double-blind phase I/II trial for metabolism-based potentiation of antimicrobial prophylaxis in the urological tract
- PMID: 39407325
- PMCID: PMC11476011
- DOI: 10.1186/s13063-024-08526-7
UROPOT: study protocol for a randomized, double-blind phase I/II trial for metabolism-based potentiation of antimicrobial prophylaxis in the urological tract
Abstract
Background: Urinary tract catheters, including Double-J or ureteral stents, are prone to bacterial colonization forming biofilms and leading to asymptomatic bacteriuria. In the context of asymptomatic bacteriuria, endourological procedures causing mucosa-inducing lesions can lead to severe infections. Antibiotic prophylaxis is warranted, yet its efficacy is limited by biofilm formation on stents. Biofilms promote antibiotic tolerance, the capacity of genetically susceptible bacteria to survive a normally lethal dose of antimicrobial therapy. The UROPOT study evaluates the effectiveness of a first-in-type metabolism-based aminoglycoside potentiation for (i) preventing infectious complications of asymptomatic bacteriuria during mucosa lesion-inducing endourological procedures and (ii) assessing its anti-tolerance efficacy.
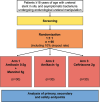
Methods: The UROPOT trial is a phase I/II single-center (Lausanne University Hospital (CHUV), Switzerland) randomized double-blinded trial. Over 2 years, patients with asymptomatic Escherichia coli and/or Klebsiella pneumoniae bacteriuria, undergoing endourological procedures, will be randomly allocated to one of three treatment arms (1:1:1 randomization ratio, 30 patients per group) to evaluate the efficacy of mannitol-potentiated low-dose amikacin compared to established standard treatments (ceftriaxone or amikacin standard dose). Patients will be recruited at the CHUV Urology Outpatient Clinic. The primary outcome is the comparative incidence of postoperative urinary tract infections (assessed at 48 h) between the investigational amikacin/mannitol therapy and standard (ceftriaxone or amikacin) antibiotic prophylaxis, defined by specific systemic symptoms and/or positive blood and/or urine culture. Secondary outcomes include assessing microbiological eradication through anti-biofilm activity, sustained microbiological eradication, and mannitol and antibiotics pharmacokinetics in blood and urine. Safety outcomes will evaluate the incidence of adverse events following amikacin/mannitol therapy and postoperative surgical complications at postoperative day 14.
Discussion: UROPOT tests a novel antimicrobial strategy based on "metabolic potentiation" for prophylaxis enabling aminoglycoside dose reduction and targeting biofilm activity. The anti-biofilm effect may prove beneficial, particularly in patients who have a permanent stent in situ needing recurrent endourological manipulations strategies in preventing infections and achieving sustained microbiological eradication in pre-stented patients.
Trial registration: The protocol is approved by the local ethics committee (CER-VD, 2023-01369, protocole 2.0) and the Swiss Agency for Therapeutic Products (Swissmedic, 701,676) and is registered on the NIH's ClinicalTrials.gov (trial registration number: NCT05761405). Registered on March 07, 2023.
Keywords: Antimicrobial prophylaxis; Biofilm; Endourological procedures; Postoperative infections; Potentiated aminoglycosides.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–60. 10.1038/nrurol.2010.190. - PubMed
-
- Bhojani N, Miller LE, Bhattacharyya S, Cutone B, Chew BH. Risk factors for urosepsis after ureteroscopy for stone disease: a systematic review with meta-analysis. J Endourol. 2021;35(7):991–1000. 10.1089/end.2020.1133. - PubMed
-
- Wollin DA, Joyce AD, Gupta M, et al. Antibiotic use and the prevention and management of infectious complications in stone disease. World J Urol. 2017;35(9):1369–79. 10.1007/s00345-017-2005-9. - PubMed
-
- Rosenberg BH, Bianco FJ, Wood DP, Triest JA. Stent-change therapy in advanced malignancies with ureteral obstruction. J Endourol. 2005;19(1):63–7. 10.1089/end.2005.19.63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical