Development and Evaluation of a Cost-Effective, Carbon-Based, Extended-Release Febuxostat Tablet
- PMID: 39407557
- PMCID: PMC11477609
- DOI: 10.3390/molecules29194629
Development and Evaluation of a Cost-Effective, Carbon-Based, Extended-Release Febuxostat Tablet
Abstract
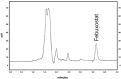
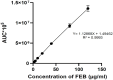
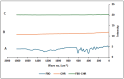
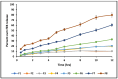
This study outlines the development of a cost-effective, extended-release febuxostat (FEB) tablet using activated charcoal as an adsorbent to enhance drug release. FEB, a BCS Class II drug, presents formulation challenges due to low solubility and high lipophilicity. We evaluated eight formulations with varying FEB-to-charcoal ratios using FTIR and DSC for physical interactions and followed USP standards for overall assessment. The optimal 1:0.25 FEB-to-charcoal ratio demonstrated a consistent 12 h zero-order release pattern. In vivo studies indicated a significantly extended plasma profile compared to immediate-release tablets. The optimal tablets demonstrated acceptable hardness and disintegration times. This innovative approach enhances patient compliance, improves bioavailability, and reduces production costs, offering a promising solution for controlled FEB delivery.
Keywords: FEB; bioavailability; charcoal; extended release; tablet.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Gerriets V., Patel P., Jialal I. Febuxostat. StatPearls; St. Petersburg, FL, USA: 2024. - PubMed
-
- Patel A.M., Patel S.R. Enhancing Solubility of Polymer-Loaded Febuxostat through Ultrasound-Assisted Microfluidic Antisolvent Nanoprecipitation: Optimization Using Box-Behnken Design. Chem. Eng. Process.—Process Intensif. 2024;201:109802. doi: 10.1016/j.cep.2024.109802. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

