1,2,4-Oxadiazole Derivatives: Physicochemical Properties, Antileishmanial Potential, Docking and Molecular Dynamic Simulations of Leishmania infantum Target Proteins
- PMID: 39407583
- PMCID: PMC11478322
- DOI: 10.3390/molecules29194654
1,2,4-Oxadiazole Derivatives: Physicochemical Properties, Antileishmanial Potential, Docking and Molecular Dynamic Simulations of Leishmania infantum Target Proteins
Abstract
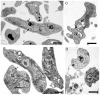
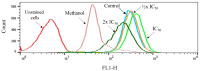
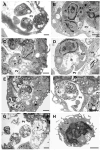
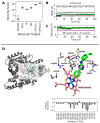
Visceral leishmaniasis (VL), caused by protozoa of the genus Leishmania, remains a significant public health concern due to its potentially lethal nature if untreated. Current chemotherapy options are limited by severe toxicity and drug resistance. Derivatives of 1,2,4-oxadiazole have emerged as promising drug candidates due to their broad biological activity. This study investigated the effects of novel 1,2,4-oxadiazole derivatives (Ox1-Ox7) on Leishmania infantum, the etiological agent of VL. In silico predictions using SwissADME suggest that these compounds have high oral absorption and good bioavailability. Among them, Ox1 showed the most promise, with higher selectivity against promastigotes and lower cytotoxicity towards L929 fibroblasts and J774.G8 macrophages. Ox1 exhibited selectivity indices of 18.7 and 61.7 against L. infantum promastigotes and amastigotes, respectively, compared to peritoneal macrophages. Ultrastructural analyses revealed severe morphological damage in both parasite forms, leading to cell death. Additionally, Ox1 decreased the mitochondrial membrane potential in promastigotes, as shown by flow cytometry. Molecular docking and dynamic simulations indicated a strong affinity of Ox1 for the L. infantum CYP51 enzyme. Overall, Ox1 is a promising and effective compound against L. infantum.
Keywords: 1,2,4-oxadiazole; chemotherapy; molecular docking; molecular dynamic; ultrastructure; visceral leishmaniasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
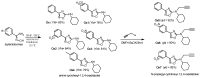
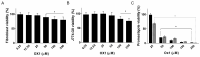
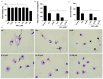
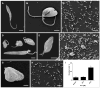
References
-
- Wamai R.G., Kahn J., McGloin J., Ziaggi G. Visceral Leishmaniasis: A Global Overview. J. Glob. Health Sci. 2020;2:e3. doi: 10.35500/jghs.2020.2.e3. - DOI
-
- Soni V., Chandel S., Pandey V., Asati S., Jain P., Jain A., Tekade R.K. Chapter 8—Novel Therapeutic Approaches for the Treatment of Leishmaniasis. In: Tekade R.K., editor. Biomaterials and Bionanotechnology. Academic Press; Cambridge, MA, USA: 2019. pp. 263–300. Advances in Pharmaceutical Product Development and Research.
-
- WHO Leishmaniasis. [(accessed on 29 June 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

