Synthetic Routes to 2-aryl-1 H-pyrrolo[2,3- b]pyridin-4-amines: Cross-Coupling and Challenges in SEM-Deprotection
- PMID: 39407670
- PMCID: PMC11478076
- DOI: 10.3390/molecules29194743
Synthetic Routes to 2-aryl-1 H-pyrrolo[2,3- b]pyridin-4-amines: Cross-Coupling and Challenges in SEM-Deprotection
Abstract
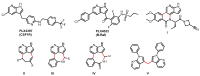
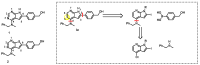
7-Azaindoles are compounds of considerable medicinal interest. During development of the structure-activity relationship for inhibitors of the colony stimulated factor 1 receptor tyrosine kinase (CSF1R), a specific 2-aryl-1H-pyrrolo[2,3-b]pyridin-4-amine was needed. Two different synthetic strategies were evaluated, in which the order of the key C-C and C-N cross-coupling steps differed. The best route relied on a chemoselective Suzuki-Miyaura cross-coupling at C-2 on a 2-iodo-4-chloropyrrolopyridine intermediate, and subsequently a Buchwald-Hartwig amination with a secondary amine at C-4. Masking of hydroxyl and pyrroles proved essential to succeed with the latter transformation. The final trimethylsilylethoxymethyl (SEM) deprotection step was challenging, as release of formaldehyde gave rise to different side products, most interestingly a tricyclic eight-membered 7-azaindole. The target 2-aryl-1H-pyrrolo[2,3-b]pyridin-4-amine (compound 3c) proved to be 20-fold less potent than the reference inhibitor, confirming the importance of the N-3 in the pyrrolopyrimidine parent compound for efficient CSF1R inhibition.
Keywords: 8-membered 7-azaindole; Buchwald–Hartwig amination; CSF1R; SEM-deprotection; azaindole; chemoselective Suzuki–Miyaura.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

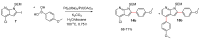

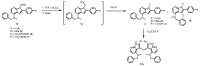
References
-
- Smirnov A.V., English D.S., Rich R.L., Lane J., Teyton L., Schwabacher A.W., Luo S., Thornburg R.W., Petrich J.W. Photophysics and Biological Applications of 7-Azaindole and Its Analogs. J. Phys. Chem. B. 1997;101:2758–2769. doi: 10.1021/jp9630232. - DOI
-
- Herrera-Ochoa D., Llano I., Ripoll C., Cybulski P., Kreuzer M., Rocha S., García-Frutos E.M., Bravo I., Garzón-Ruiz A. Protein aggregation monitoring in cells under oxidative stress: A novel fluorescent probe based on a 7-azaindole-BODIPY derivative. J. Mater. Chem. B. 2024;12:7577–7590. doi: 10.1039/D4TB00567H. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

