Lenvatinib Plus Pembrolizumab versus Doxorubicin for Advanced or Recurrent Endometrial Cancer with Short Treatment-Free Intervals Following First-Line Carboplatin Plus Paclitaxel
- PMID: 39407730
- PMCID: PMC11476733
- DOI: 10.3390/jcm13195670
Lenvatinib Plus Pembrolizumab versus Doxorubicin for Advanced or Recurrent Endometrial Cancer with Short Treatment-Free Intervals Following First-Line Carboplatin Plus Paclitaxel
Abstract
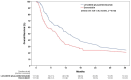
Background: The treatment-free interval is a significant predictor of worse prognosis and poor response rates of the second-line treatment in patients with carboplatin and paclitaxel (PT)-pretreated, advanced, or recurrent endometrial cancer (EC). Whether lenvatinib plus pembrolizumab still confers a survival benefit compared with doxorubicin in patients with platinum-free intervals of <6 months remains unclear. Methods: This multi-institutional retrospective analysis was performed using de-identified electronic health records from the TriNetX Research Network. Patients with advanced or recurrent ECs who received lenvatinib plus pembrolizumab or doxorubicin within six months of first-line PT were identified. A 1:1 propensity score matching (PSM) was conducted to control for potential confounding variables. Overall survival (OS) and adverse event profile were the primary and secondary outcomes. Results: Between January 2018 and February 2024, 130 patients with PT-treated, advanced, or recurrent ECs who received lenvatinib plus pembrolizumab and 122 patients who received doxorubicin at a platinum-free interval of <6 months were identified across 31 healthcare organizations. In the balanced cohort following PSM with 117 patients in each group, treatment with lenvatinib plus pembrolizumab was associated with improved OS compared with treatment with doxorubicin (12.8 vs. 8.2 months, p = 0.012, hazard ratio: 0.65, 95% confidence interval: 0.46-0.91). Regarding adverse event analysis, a higher incidence of hypothyroidism and proteinuria was observed with lenvatinib plus pembrolizumab, and more hematological toxicities were observed with doxorubicin. Conclusions: in patients with treatment-free intervals of <6 months, lenvatinib plus pembrolizumab still confers improved survival compared with doxorubicin in PT-treated, advanced, or recurrent ECs.
Keywords: advanced endometrial cancer; adverse events; doxorubicin; lenvatinib; pembrolizumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Miller D.S., Filiaci V.L., Mannel R.S., Cohn D.E., Matsumoto T., Tewari K.S., DiSilvestro P., Pearl M.L., Argenta P.A., Powell M.A., et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209) J. Clin. Oncol. 2020;38:3841–3850. doi: 10.1200/JCO.20.01076. - DOI - PMC - PubMed
-
- Nagao S., Nishio S., Michimae H., Tanabe H., Okada S., Otsuki T., Tanioka M., Fujiwara K., Suzuki M., Kigawa J. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: The SGSG-012/GOTIC-004/Intergroup study. Gynecol. Oncol. 2013;131:567–573. doi: 10.1016/j.ygyno.2013.09.021. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous