Clinical Expression of Familial Hypercholesterolemia in Patients from France and French Canada Carrying Identical-by-Descent Pathogenic LDLR Gene Variants: A Proof-of-Concept Study
- PMID: 39407785
- PMCID: PMC11477318
- DOI: 10.3390/jcm13195725
Clinical Expression of Familial Hypercholesterolemia in Patients from France and French Canada Carrying Identical-by-Descent Pathogenic LDLR Gene Variants: A Proof-of-Concept Study
Abstract
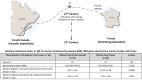
Background: Studying patients carrying identical-by-descent (IBD) pathogenic gene variants allows us to control for the disease-causing genetic background and to more accurately document the impact of modifiers. Familial hypercholesterolemia (FH) is characterized by elevated low-density lipoprotein cholesterol (LDL-c) levels and premature atherosclerosis and is often caused by defects in the LDLR gene. There is a high prevalence of FH in French Canada as a result of a founder effect from France in the 17th century. Several FH patients currently living in French Canada (founder population) and in France (colonizing population) carry IBD FH-causing variants. The expression of FH is affected by environmental and genetic modifiers, and patients with IBD variants may present different characteristics. Methods: In this study, we compared FH clinical expression patients carrying IBD LDLR pathogenic variants living in France or Canada. Four IBD variants, namely c.259T>G p.(Trp87Gly), c.2000G>A p.(Cys667Tyr), c.682G>A p.(Glu228Lys), and c.1048C>T p.(Arg350*), were selected. Untreated plasma lipid profiles, the apolipoprotein E (APOE) genotype, cardiovascular risk factors, and the occurrence of symptomatic ASCVD were compared in 105 adult carriers (30 from France and 75 from French Canada). Results: All parameters were similar between the two populations, except for untreated total cholesterol (10.14 ± 1.89 mmol/L vs. 8.65 ± 1.84 mmol/L, p = 0.0006) and LDL-c concentrations (7.94 ± 1.86 mmol/L vs. 6.93 ± 1.78 mmol/L, p = 0.016), which were significantly higher in FH patients living in France, an observation that was revealed across all studied LDLR variants. Conclusions: This study illustrates that FH patients sharing IBD pathogenic LDLR variants that have evolved in different geographic, cultural, and socio-economic environments for hundreds of years differ in terms of cholesterol levels, highlighting the importance of better understanding the interplay between genetic and environmental modulators of FH expression.
Keywords: familial hypercholesterolemia; founder effect; identical-by-descent variant.
Conflict of interest statement
The authors have no conflict of interest to disclose in this study.
Figures
Similar articles
-
ABCG5 and ABCG8 genetic variants in familial hypercholesterolemia.J Clin Lipidol. 2020 Mar-Apr;14(2):207-217.e7. doi: 10.1016/j.jacl.2020.01.007. Epub 2020 Jan 29. J Clin Lipidol. 2020. PMID: 32088153
-
DIAgnosis and Management Of familial hypercholesterolemia in a Nationwide Design (DIAMOND-FH): Prevalence in Switzerland, clinical characteristics and the diagnostic value of clinical scores.Atherosclerosis. 2018 Oct;277:282-288. doi: 10.1016/j.atherosclerosis.2018.08.009. Atherosclerosis. 2018. PMID: 30270060
-
Low-density lipoprotein apheresis: an evidence-based analysis.Ont Health Technol Assess Ser. 2007;7(5):1-101. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2007. PMID: 23074505 Free PMC article.
-
The molecular genetic basis and diagnosis of familial hypercholesterolemia in Denmark.Dan Med Bull. 2002 Nov;49(4):318-45. Dan Med Bull. 2002. PMID: 12553167 Review.
-
Genetic testing for familial hypercholesterolemia: Impact on diagnosis, treatment and cardiovascular risk.Eur J Prev Cardiol. 2019 Aug;26(12):1262-1270. doi: 10.1177/2047487319829746. Epub 2019 Feb 12. Eur J Prev Cardiol. 2019. PMID: 30755017
References
-
- Goldstein J.L., Hobbs H.H., Brown M.S. Familial Hypercholesterolemia. In: Valle D.L., Antonarakis S., Ballabio A., Beaudet A.L., Mitchell G.A., editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Education; New York, NY, USA: 2019.
-
- Cuchel M., Raal F.J., Hegele R.A., Al-Rasadi K., Arca M., Averna M., Bruckert E., Freiberger T., Gaudet D., Harada-Shiba M., et al. 2023 Update on European Atherosclerosis Society Consensus Statement on Homozygous Familial Hypercholesterolaemia: New treatments and clinical guidance. Eur. Heart J. 2023;44:2277–2291. doi: 10.1093/eurheartj/ehad197. - DOI - PMC - PubMed
-
- Santos R.D., Gidding S.S., Hegele R.A., Cuchel M.A., Barter P.J., Watts G.F., Baum S.J., Catapano A.L., Chapman M.J., Defesche J.C., et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4:850–861. doi: 10.1016/S2213-8587(16)30041-9. - DOI - PubMed
-
- Safarova M.S., Santos R.D., Moriarty P.M. 34—Special Patient Populations: Familial Hypercholesterolemia and Other Severe Hypercholesterolemias. In: Ballantyne C.M., editor. Clinical Lipidology. 3rd ed. Elsevier; New Delhi, India: 2024. pp. 320–335.e2. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

