Combined Effects of Cyclic Hypoxic and Mechanical Stimuli on Human Bone Marrow Mesenchymal Stem Cell Differentiation: A New Approach to the Treatment of Bone Loss
- PMID: 39407866
- PMCID: PMC11476683
- DOI: 10.3390/jcm13195805
Combined Effects of Cyclic Hypoxic and Mechanical Stimuli on Human Bone Marrow Mesenchymal Stem Cell Differentiation: A New Approach to the Treatment of Bone Loss
Abstract
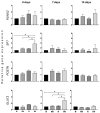
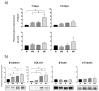
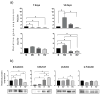
Background: The prevention and treatment of bone loss and osteoporotic fractures is a public health challenge. Combined with normobaric hypoxia, whole-body vibration has a high clinic potential in bone health and body composition. The effect of this therapy may be mediated by its action on bone marrow mesenchymal stem cells (MSCs). Objectives: Evaluate the effects of cyclic low-vibration stimuli and/or hypoxia on bone marrow-derived human MSC differentiation. Methods: MSCs were exposed four days per week, two hours/day, to hypoxia (3% O2) and/or vibration before they were induced to differentiate or during differentiation into osteoblasts or adipocytes. Gene and protein expression of osteoblastic, adipogenic, and cytoskeletal markers were studied, as well as extracellular matrix mineralization and lipid accumulation. Results: early osteoblastic markers increased in undifferentiated MSCs, pretreated in hypoxia and vibration. This pretreatment also increased mRNA levels of osteoblastic genes and beta-catenin protein in the early stages of differentiation into osteoblasts without increasing mineralization. When MSCs were exposed to vibration under hypoxia or normoxia during osteoblastic differentiation, mineralization increased with respect to cultures without vibrational stimuli. In MSCs differentiated into adipocytes, both in those pretreated as well as exposed to different conditions during differentiation, lipid formation decreased. Changes in adipogenic gene expression and increased beta-catenin protein were observed in cultures treated during differentiation. Conclusions: Exposure to cyclic hypoxia in combination with low-intensity vibratory stimuli had positive effects on osteoblastic differentiation and negative ones on adipogenesis of bone marrow-derived MSCs. These results suggest that in elderly or frail people with difficulty performing physical activity, exposure to normobaric cyclic hypoxia and low-density vibratory stimuli could improve bone metabolism and health.
Keywords: adipocyte; bone; hypoxia; mechanical stimuli; mesenchymal stem cells; osteoblast.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources

