Monoclonal Antibodies for the Treatment of Ocular Diseases
- PMID: 39407875
- PMCID: PMC11482488
- DOI: 10.3390/jcm13195815
Monoclonal Antibodies for the Treatment of Ocular Diseases
Abstract
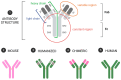
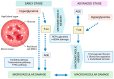
Monoclonal antibodies (mAbs) have revolutionized the landscape of cancer therapy, offering unprecedented specificity and diverse mechanisms to combat malignant cells. These biologic agents have emerged as a cornerstone in targeted cancer treatment, binding to specific antigens on cancer cells and exerting their therapeutic effects through various mechanisms, including inhibition of signaling pathways, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP). The unique ability of mAbs to engage the immune system and directly interfere with cancer cell function has significantly enhanced the therapeutic armamentarium against a broad spectrum of malignancies. mAbs were initially studied in oncology; however, today, treatments have been developed for eye diseases. This review discusses the current applications of mAbs for the treatment of ocular diseases, discussing the specificity and the variety of mechanisms by which these molecules exhibit their therapeutic effects. The benefits, drawbacks, effectiveness, and risks associated with using mAbs in ophthalmology are highlighted, focusing on the most relevant ocular diseases and mAbs currently in use. Technological advances have led to in vitro production methods and recombinant engineering techniques, allowing the development of chimeric, humanized, and fully human mAbs. Nowadays, many humanized mAbs have several applications, e.g., for the treatment of age-related macular disease, diabetic retinopathy, and uveitis, while studies about new applications of mAbs, such as for SARS-CoV-2 infection, are also currently ongoing to seek more efficient and safe approaches to treat this new ocular disease.
Keywords: age-related macular degeneration; diabetic retinopathy; monoclonal antibodies; ocular diseases; uveitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Rodríguez-Nava C., Ortuño-Pineda C., Illades-Aguiar B., Flores-Alfaro E., Leyva-Vázquez M.A., Parra-Rojas I., del Moral-Hernández O., Vences-Velázquez A., Cortés-Sarabia K., Alarcón-Romero L.d.C. Mechanisms of action and limitations of monoclonal antibodies and single chain fragment variable (scFv) in the treatment of cancer. Biomedicines. 2023;11:1610. doi: 10.3390/biomedicines11061610. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

