Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study
- PMID: 39408026
- PMCID: PMC11477643
- DOI: 10.3390/jcm13195966
Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study
Abstract
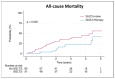
Background: Patients with transthyretin amyloid cardiomyopathy (ATTR-CM) represent a high-risk heart failure population with continued unmet therapeutic needs. Sodium-glucose co-transporter 2 inhibitors (SGLT2i) improve cardiovascular outcomes in patients with heart failure across the whole spectrum of ejection fraction, and first evidence regarding their safety and effectiveness in patients with ATTR-CM is arising. This study investigates the association between SGLT2i therapy and clinical outcomes in these patients. Methods: This is an analysis of a prospective registry conducted at a referral centre for hypertrophic cardiomyopathies including 116 patients with confirmed ATTR-CM. Fifty-one patients (44%) were treated with SGLT2i while 65 patients (56%) remained SGLT2i-naïve. Results: During a median follow-up of 2.6 (1.7-3.7) years, 38 patients (33%) died, of whom 11 patients (9%) received SGLT2i treatment and 27 patients (23%) were treatment-naïve. SGLT2i therapy was significantly associated with lower mortality (HR 0.457, 95%CI 0.227-0.922, p = 0.029). This association persisted after adjusting for age and sex (HR 0.479, 95%CI 0.235-0.977, p = 0.043) and after additional adjustment for eGFR, NT-proBNP, LVEF, and concomitant therapy with tafamidis (HR 0.328, 95%CI 0.141-0.760, p = 0.009). However, when potential immortal time bias was considered, this association lost statistical significance (HR 1.075, 95%CI 0.524-2.206, p = 0.843). No significant associations between SGLT2i therapy and worsening heart-failure hospitalization or cardiovascular mortality were observed. Conclusions: In crude analysis, SGLT2i therapy associates with better survival in patients with ATTR-CM. However, after adjustment for immortal time, this association becomes statistically insignificant. Hence, to draw final conclusions on the effectiveness of SGLT2i therapy in these patients, a randomized controlled trial is warranted.
Keywords: Sodium–glucose co-transporter 2 inhibitors; heart failure therapy; survival; transthyretin amyloid cardiomyopathy.
Conflict of interest statement
H.S. is on the advisory board and speakers bureau of Boehringer Ingelheim, NovoNordisk, Amgen, AstraZeneca, Bayer, Eli Lilly, Cancom, MSD, and Daiichi Sankyo. N.V. has received an unrestricted grant from Boehringer Ingelheim. All other authors report no conflicts of interest.
Figures
Similar articles
-
Sodium-glucose cotransporter 2 inhibitors and outcomes in transthyretin amyloid cardiomyopathy: Systematic review and meta-analysis.Eur J Clin Invest. 2025 Jun;55(6):e14392. doi: 10.1111/eci.14392. Epub 2025 Jan 27. Eur J Clin Invest. 2025. PMID: 39868862 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors in transthyretin amyloid cardiomyopathy: navigating potential benefits and uncertainties.Curr Med Res Opin. 2025 Apr;41(4):657-661. doi: 10.1080/03007995.2025.2495167. Epub 2025 Apr 24. Curr Med Res Opin. 2025. PMID: 40249203 Review.
-
SGLT2 Inhibitor Therapy in Patients With Transthyretin Amyloid Cardiomyopathy.J Am Coll Cardiol. 2024 Jun 18;83(24):2411-2422. doi: 10.1016/j.jacc.2024.03.429. J Am Coll Cardiol. 2024. PMID: 38866445
-
Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Cardiovascular Outcomes in Transthyretin Amyloid Cardiomyopathy.Am J Cardiol. 2025 May 15;243:15-18. doi: 10.1016/j.amjcard.2025.01.012. Epub 2025 Feb 6. Am J Cardiol. 2025. PMID: 39921099
-
SGLT2 inhibitor therapy for transthyretin amyloid cardiomyopathy: early tolerance and clinical response to dapagliflozin.ESC Heart Fail. 2023 Feb;10(1):397-404. doi: 10.1002/ehf2.14188. Epub 2022 Oct 19. ESC Heart Fail. 2023. PMID: 36259276 Free PMC article.
Cited by
-
Tolerability and efficacy of sodium-glucose co-transporter 2 inhibitors in patients with cardiac amyloidosis: a meta-analysis of observational studies.Eur Heart J Cardiovasc Pharmacother. 2025 Jul 7;11(4):356-364. doi: 10.1093/ehjcvp/pvaf033. Eur Heart J Cardiovasc Pharmacother. 2025. PMID: 40380981 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors and outcomes in transthyretin amyloid cardiomyopathy: Systematic review and meta-analysis.Eur J Clin Invest. 2025 Jun;55(6):e14392. doi: 10.1111/eci.14392. Epub 2025 Jan 27. Eur J Clin Invest. 2025. PMID: 39868862 Free PMC article.
References
-
- Lane T., Fontana M., Martinez-Naharro A., Quarta C.C., Whelan C.J., Petrie A., Rowczenio D.M., Gilbertson J.A., Hutt D.F., Rezk T., et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation. 2019;140:16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. - DOI - PubMed
-
- Lohrmann G., Pipilas A., Mussinelli R., Gopal D.M., Berk J.L., Connors L.H., Vellanki N., Hellawell J., Siddiqi O.K., Fox J., et al. Stabilization of Cardiac Function With Diflunisal in Transthyretin (ATTR) Cardiac Amyloidosis. J. Card. Fail. 2020;26:753–759. doi: 10.1016/j.cardfail.2019.11.024. - DOI - PMC - PubMed
-
- McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A., Ponikowski P., Sabatine M.S., Anand I.S., Belohlavek J., et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

