Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment
- PMID: 39408046
- PMCID: PMC11477653
- DOI: 10.3390/jcm13195986
Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment
Abstract
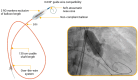
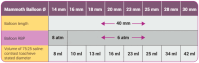
Background: Balloon aortic valvuloplasty (BAV) is currently used as pre-treatment for patients undergoing trans-catheter aortic valve replacement (TAVR) as well as a stand-alone option for subjects with significant contraindications to TAVR. Mammoth is a newly available non-compliant balloon catheter (BC) included in the balloon-expandable Myval THV system (Meril Life Sciences Pvt. Ltd., India). As limited data on the performance of this BC are available, we here report the results following its use for BAV as pre-dilatation during TAVR or as a stand-alone procedure. Methods: A retrospective, single-center cohort analysis was performed on patients with severe aortic valve stenosis (AS) treated with the Mammoth BC at IRCCS Ospedale Galeazzi Sant'Ambrogio, Milan, Italy. The primary endpoint was technical success defined as successful Mammoth BC advancement across the AS followed by its full and homogeneous inflation without major complications such as aortic root/left ventricular outflow tract injury and/or stroke. Results: A total of 121 patients were treated by BAV with Mammoth BC during the study period. Among these, 105 patients underwent BAV pre-dilatation before TAVR while 16 patients underwent a stand-alone BAV procedure. Mammoth BC was delivered and successfully inflated at the target site in all of the 121 cases without BC-related complications (100% technical success). However, in the BAV "stand-alone group", three patients required two different balloon sizes while in nine patients multiple rounds (two to three) of balloon inflation were needed to significantly lower the transvalvular gradient. No cases of aortic root injury or massive aortic regurgitation due to Mammoth BC-related aortic leaflet injury were reported while one major stroke occurred late after TAVR. No intra-procedural deaths occurred nor bleeding (BARC 3-4) or major vascular complication. Conclusions: Mammoth BC use in patients with severe AS proved safe and effective, either before TAVR or as a stand-alone procedure, expanding the range of available tools for structural operators.
Keywords: aortic valve stenosis; balloon aortic valvuloplasty; balloon catheter; trans-catheter aortic valve replacement.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Ternacle J., Al-Azizi K., Szerlip M., Potluri S., Hamandi M., Blanke P., Leipsic J., Dahou A., Salaun E., Vincent F., et al. Impact of Predilation During Transcatheter Aortic Valve Replacement: Insights from the PARTNER 3 Trial. Circ. Cardiovasc. Interv. 2021;14:7. doi: 10.1161/CIRCINTERVENTIONS.120.010336. - DOI - PubMed
-
- Husser O., Pellegrini C., Kessler T., Burgdorf C., Thaller H., Mayr N.P., Kasel A.M., Kastrati A., Schunkert H., Hengstenberg C. Predictors of Permanent Pacemaker Implantations and New-Onset Conduction Abnormalities with the SAPIEN 3 Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc. Interv. 2016;9:244–254. doi: 10.1016/j.jcin.2015.09.036. - DOI - PubMed
-
- Meredith I.T., Walters D.L., Dumonteil N., Worthley S.G., Tchétché D., Manoharan G., Blackman D.J., Rioufol G., Hildick-Smith D., Whitbourn R.J., et al. 1-Year Outcomes with the Fully Repositionable and Retrievable Lotus Transcatheter Aortic Replacement Valve in 120 High-Risk Surgical Patients with Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2016;9:376–384. doi: 10.1016/j.jcin.2015.10.024. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials