Estetrol Inhibits the Prostate Cancer Tumor Stimulators FSH and IGF-1
- PMID: 39408055
- PMCID: PMC11478095
- DOI: 10.3390/jcm13195996
Estetrol Inhibits the Prostate Cancer Tumor Stimulators FSH and IGF-1
Abstract
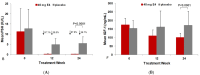
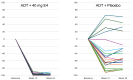
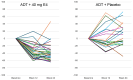
Background: The co-treatment of androgen deprivation therapy (ADT) for advanced prostate cancer (PCa) with the fetal estrogen estetrol (E4) may further inhibit endocrine PCa tumor stimulators. We previously reported the suppression of follicle-stimulating hormone (FSH), total and free testosterone, and prostate-specific antigen by ADT+E4. Here, we provide more detailed data on FSH suppression by E4 and present new findings on the effect of ADT+E4 on insulin-like growth factor-1 (IGF-1). Methods: A Phase II, double-blind, randomized, placebo-controlled study (the PCombi study) was conducted in advanced PCa patients treated with ADT. The study assessed the effect of E4 co-treatment with LHRH agonist ADT on tumor stimulators, including FSH and IGF-1. Patients starting ADT were randomized 2:1 to receive either 40 mg E4 (n = 41) or placebo (n = 21) for 24 weeks. Non-parametric analyses were performed on the per-protocol population (PP) and individual changes were visualized. Results: The PP included 57 patients (37 ADT+E4; 20 ADT+placebo). ADT+E4 almost completely suppressed FSH in all patients (98% versus 37%; p < 0.0001). IGF-1 levels decreased by 41% with ADT+E4 versus an increase of 10% with ADT+placebo (p < 0.0001). Conclusions: The almost complete suppression of the tumor stimulator FSH using ADT plus E4 observed in all individual patients in this study, along with the augmented suppression of IGF-1 versus an increase by ADT only, may be clinically relevant and suggest the enhanced anti-cancer treatment efficacy of E4 in addition to the previously reported additional suppression of total and free T and PSA.
Keywords: PCombi study; androgen deprivation therapy (ADT); estetrol (E4); follicle-stimulating hormone (FSH); insulin-like growth factor-1 (IGF-1).
Conflict of interest statement
H. Coelingh Bennink has retired as president of Pantarhei Bioscience and its affiliate Pantarhei Oncology. I. J. Schultz is R&D director, and J. Krijgh is chief medical officer of Pantarhei Oncology. R. J. van Moorselaar received grants and fees from Astellas, Ipsen, Astra Zeneca, and Bayer and Janssen. D. Somford is a member of the advisory boards of Astellas and Janssen and received a research grant from Astellas. The other authors declare no conflicts of interest.
Figures
References
-
- Gillessen S., Bossi A., Davis I.D., de Bono J., Fizazi K., James N.D., Mottet N., Shore N., Small E., Smith M., et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur. J. Cancer. 2023;185:178–215. doi: 10.1016/j.ejca.2023.02.018. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

