Management of Busulfan-Induced Lung Injury in Pediatric Patients with High-Risk Neuroblastoma
- PMID: 39408056
- PMCID: PMC11477708
- DOI: 10.3390/jcm13195995
Management of Busulfan-Induced Lung Injury in Pediatric Patients with High-Risk Neuroblastoma
Abstract
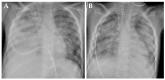
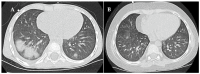
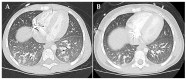
Background/Objectives: Integrating the cytotoxic drug busulfan into a high-dose chemotherapy regimen prior to autologous hematopoietic stem cell rescue in patients with high-risk neuroblastoma has improved the survival of children battling this deadly disease. Busulfan-induced toxicities can, however, be severe. Here, we describe the diagnosis and successful treatment of acute pulmonary injury by total-body-weight-adjusted busulfan therapy in two children with high-risk neuroblastoma. Case series: Patient 1 developed life-threatening biphasic acute respiratory failure on days +60 and +100 after busulfan therapy, requiring intubation and invasive mechanical ventilation. Despite intensive anti-inflammatory and immunomodulatory therapy, including systemic corticosteroids, topical inhalation regimens, azithromycin, nintedanib and extracorporal photopheresis, patient 1 required extended intensive care measures and non-invasive respiratory support for a total of 20 months. High-resolution computed tomography showed diffuse intra-alveolar and interstitial patterns. Patient 2 developed partial respiratory failure with insufficient oxygen saturation and dyspnea on day +52 after busulfan therapy. Symptoms were resolved after 6 months of systemic corticosteroids, topical inhalation regimens and azithromycin. High-resolution computed tomography showed atypical pneumonic changes with ground-glass opacities. While both patients fully recovered without evidence of pulmonary fibrosis, cancer therapy had to be paused and then modified until full recovery from busulfan-induced lung injury. Conclusions: Busulfan-induced lung injury requires prompt diagnosis and intervention. Symptoms and signs are nonspecific and difficult to differentiate from other causes. Therapeutic busulfan drug level monitoring and the identification of patients at risk for drug overdosing through promoter polymorphisms in the glutathione S-transferase alpha 1 gene encoding the main enzyme in busulfan metabolism are expected to reduce the risk of busulfan-induced toxicities.
Keywords: busulfan; busulfan-induced lung injury; high-dose chemotherapy; neuroblastoma; pediatric cancer; pharmacogenomics; restrictive lung disease; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Busulfan-Induced Lung Injury in Pediatric Oncology Patients-Review of the Literature with an Illustrative Case.Pediatr Allergy Immunol Pulmonol. 2019 Sep 1;32(3):86-91. doi: 10.1089/ped.2019.0990. Epub 2019 Sep 17. Pediatr Allergy Immunol Pulmonol. 2019. PMID: 32140277 Free PMC article. Review.
-
[Chinese experts consensus statement: diagnosis and treatment of cystic fibrosis (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2023 Apr 12;46(4):352-372. doi: 10.3760/cma.j.cn112147-20221214-00971. Zhonghua Jie He He Hu Xi Za Zhi. 2023. PMID: 36990700 Chinese.
-
Idiopathic pneumonia syndrome following myeloablative chemotherapy and autologous transplantation.Ann Pharmacother. 2001 Feb;35(2):196-201. doi: 10.1345/aph.10071. Ann Pharmacother. 2001. PMID: 11215840
-
[Busulfan lung exacerbated during steroid therapy: a review of Japanese literature].Rinsho Ketsueki. 1990 Nov;31(11):1884-8. Rinsho Ketsueki. 1990. PMID: 2287077 Review. Japanese.
References
-
- Hartmann O., Valteau-Couanet D., Vassal G., Lapierre V., Brugières L., Delgado R., Couanet D., Lumbroso J., Benhamou E. Prognostic factors in metastatic neuroblastoma in patients over 1 year of age treated with high-dose chemotherapy and stem cell transplantation: A multivariate analysis in 218 patients treated in a single institution. Bone Marrow Transplant. 1999;23:789–795. doi: 10.1038/sj.bmt.1701737. - DOI - PubMed
-
- Proust-Houdemont S., Pasqualini C., Blanchard P., Dufour C., Benhamou E., Goma G., Semeraro M., Raquin M.-A., Hartmann O., Valteau-Couanet D. Busulfan-melphalan in high-risk neuroblastoma: The 30-year experience of a single institution. Bone Marrow Transplant. 2016;51:1076–1081. doi: 10.1038/bmt.2016.75. - DOI - PubMed
-
- Granger M.M., Naranjo A., Bagatell R., DuBois S.G., McCune J.S., Tenney S.C., Weiss B.D., Mosse Y.P., Asgharzadeh S., Grupp S.A., et al. Myeloablative Busulfan/Melphalan Consolidation following Induction Chemotherapy for Patients with Newly Diagnosed High-Risk Neuroblastoma: Children’s Oncology Group Trial ANBL12P1. Transplant. Cell. Ther. 2021;27:490.e1–490.e8. doi: 10.1016/j.jtct.2021.03.006. - DOI - PMC - PubMed
-
- Ladenstein R., Pötschger U., Pearson A.D.J., Brock P., Luksch R., Castel V., Yaniv I., Papadakis V., Laureys G., Malis J., et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–514. doi: 10.1016/S1470-2045(17)30070-0. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

