Effect of Personalized Prebiotic and Probiotic Supplements on the Symptoms of Irritable Bowel Syndrome: An Open-Label, Single-Arm, Multicenter Clinical Trial
- PMID: 39408300
- PMCID: PMC11478705
- DOI: 10.3390/nu16193333
Effect of Personalized Prebiotic and Probiotic Supplements on the Symptoms of Irritable Bowel Syndrome: An Open-Label, Single-Arm, Multicenter Clinical Trial
Abstract
Background/objectives: Prebiotics and probiotics have been reported to improve symptoms of irritable bowel syndrome (IBS). Nevertheless, the effects of prebiotics/probiotics can vary depending on the IBS subtypes. The purpose of this study was to investigate the effects of personalized prebiotic and probiotic supplements based on intestinal microbiota and IBS subtypes in patients.
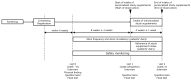
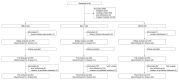
Methods: Patients with diarrhea-type IBS (IBS-D), constipation-type IBS (IBS-C), and mixed-type IBS (IBS-M) were enrolled (n = 40 per group; total: n = 120). Personalized prebiotic and probiotic supplements were determined according to the IBS subtypes and intestinal microbiota. The patients received supplements for 4 weeks. The primary outcome was the change in the IBS-severity scoring system from baseline to week 4.
Results: The IBS-severity scoring system significantly decreased in all patients (-38.0 [95% confidence interval (CI): -53.6, -22.4]; p < 0.001), in patients with IBS-D (-44.5 [95% CI: -70.6, -18.5]; p = 0.004) and IBS-C (-51.2 [95% CI: -79.4, -22.9]; p = 0.002), but not in those with IBS-M (-20.0 [95% CI: -48.0, 8.1]; p = 0.47). In this study, no serious adverse events were observed that had a causal relationship with the intervention.
Conclusions: In conclusion, personalized prebiotic and probiotic supplements selected according to individual intestinal microbiota and IBS subtype may alleviate the severity of IBS symptoms, particularly in patients with IBS-C and IBS-D.
Keywords: irritable bowel syndrome; microbiome; personalized; prebiotics; probiotics.
Conflict of interest statement
Nozomi Matsuura, Masaya Kanayama, and Yuta Watanabe are employees of Kirin Holdings Company, Limited. Loukia Lili is an employee of Thorne HealthTech, Inc. The remaining authors have no conflicts of interest to declare.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

