Comparative Study of Prebiotics for Infants Using a Fecal Culture System: Insights into Responders and Non-Responders
- PMID: 39408314
- PMCID: PMC11478422
- DOI: 10.3390/nu16193347
Comparative Study of Prebiotics for Infants Using a Fecal Culture System: Insights into Responders and Non-Responders
Abstract
Background: The gut microbiota of breast-fed infants is dominated by infant-type human-residential bifidobacteria (HRB) that contribute to infant health; thus, it is crucial to develop infant formulas that promote the establishment of a gut microbiota enriched with infant-type HRB, closely resembling that of breastfed infants.
Methods: We compared various non-digestible prebiotic oligosaccharides and their combinations using a fecal culture system to explore which candidates could promote the growth of all infant-type HRB and rarely yield non-responders. The analysis included lactulose (LAC), raffinose (RAF), galactooligosaccharides (GOS), and short- and long-chain fructooligosaccharides. Fecal samples were collected from seven infants aged 1.5-10.2 months and cultured with each oligosaccharide individually or their combinations.
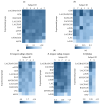
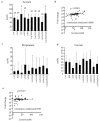
Results: No single oligosaccharide effectively promoted the growth of all infant-type HRB, although GOS promoted the growth of HRB other than Bifidobacterium longum subsp. longum. Only the LAC/RAF/GOS group evenly and effectively promoted the growth of all infant-type HRB. Accordingly, acetate production was higher in fecal cultures supplemented with GOS or LAC/RAF/GOS than in the other cultures, suggesting that it is a superior combination for all infant-type HRB and rarely yields non-responders.
Conclusions: This study can aid in developing infant formulas that help align the gut microbiota of formula-fed infants with that of breastfed infants.
Keywords: bifidobacteria; human-residential bifidobacteria; infant formula; non-digestible oligosaccharide; non-responder; prebiotics.
Conflict of interest statement
S.M., E.T., N.H. and M.K., are employed by Morinaga Milk Industry Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice.Br J Nutr. 2016 Jul;116(2):270-8. doi: 10.1017/S0007114516001987. Epub 2016 May 20. Br J Nutr. 2016. PMID: 27198516
-
Bifidogenic Effect of 2'-Fucosyllactose (2'-FL) on the Gut Microbiome of Healthy Formula-Fed Infants: A Randomized Clinical Trial.Nutrients. 2025 Mar 11;17(6):973. doi: 10.3390/nu17060973. Nutrients. 2025. PMID: 40290019 Free PMC article. Clinical Trial.
-
Fermented infant formula (with Bifidobacterium breve C50 and Streptococcus thermophilus O65) with prebiotic oligosaccharides is safe and modulates the gut microbiota towards a microbiota closer to that of breastfed infants.Clin Nutr. 2021 Mar;40(3):778-787. doi: 10.1016/j.clnu.2020.07.024. Epub 2020 Jul 23. Clin Nutr. 2021. PMID: 32893049 Clinical Trial.
-
Prebiotics in infant formulas.J Clin Gastroenterol. 2004 Jul;38(6 Suppl):S76-9. doi: 10.1097/01.mcg.0000128927.91414.93. J Clin Gastroenterol. 2004. PMID: 15220664 Review.
-
Prebiotics in infant formula.Gut Microbes. 2014;5(6):681-7. doi: 10.4161/19490976.2014.972237. Gut Microbes. 2014. PMID: 25535999 Free PMC article. Review.
References
-
- Hiraku A., Nakata S., Murata M., Xu C., Mutoh N., Arai S., Odamaki T., Iwabuchi N., Tanaka M., Tsuno T., et al. Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium Longum Subsp. Infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients. 2023;15:1402. doi: 10.3390/nu15061402. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

