The Effects of Brodalumab on the Fungal Microbiome in Patients with Psoriasis
- PMID: 39408568
- PMCID: PMC11475962
- DOI: 10.3390/ijms251910239
The Effects of Brodalumab on the Fungal Microbiome in Patients with Psoriasis
Abstract
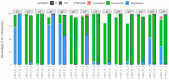
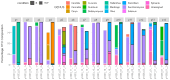
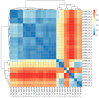
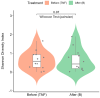
The gut microbiota plays a critical role in immune system function, with dysbiosis linked to systemic inflammation, contributing to conditions like psoriasis and depression. Although biological treatments for severe psoriasis are known to impact gut bacteria, less is understood about their effects on fungi. This study aims to investigate fungal gut microbiota changes in psoriasis patients transitioning from TNF-α inhibitors to brodalumab. Fecal samples from 20 patients were analyzed using Illumina MiSeq sequencing of the ITS2 region of 18S rRNA. Microbial diversity was assessed through Bray-Curtis dissimilarity and the Shannon-Wiener index. Clinical outcomes were measured using clinical scores for psoriasis and depression severity, with statistical analysis performed via Wilcoxon signed-rank tests and PERMANOVA. Results showed that Ascomycota was the dominant fungal phylum in both treatment groups, with Saccharomyces, Penicillium, Candida, and Debaryomyces as prevalent genera. No significant changes occurred at the phylum level after switching to brodalumab, though minor genome-level variations were observed. Beta diversity analysis highlighted inter-patient variability, with no significant correlation between fungal composition and clinical outcomes. Despite improved clinical scores, the fungal gut microbiota remained largely stable, suggesting that brodalumab does not significantly alter fungal communities in psoriasis patients. Further research is needed for a comprehensive understanding.
Keywords: TNF-alpha inhibitors; brodalumab; fungi; gut microbiome; psoriasis.
Conflict of interest statement
Amra Osmančević has participated or held lectures in AB for AbbVie, Celgene/Amgen, Eli Lilly, Novartis, Pfizer, Meda, UCB-Pharma, Janssen Cilag, Allmiral, Kyowa Kirin, Sanofi, Bristol-Myers Squbb, Recordati Rare Diseases, and Leo Pharma and conducted clinical trials for Novartis, Abbvie, Leo Pharma, Celgene, Janssen Cilag, UCB-Pharma, and Boehringer Ingelheim. Authors Yadhu Kumar and Maria Göthe were employed by the company Eurofins Genomics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

