Metabolic Engineering and Process Intensification for Muconic Acid Production Using Saccharomyces cerevisiae
- PMID: 39408575
- PMCID: PMC11476194
- DOI: 10.3390/ijms251910245
Metabolic Engineering and Process Intensification for Muconic Acid Production Using Saccharomyces cerevisiae
Abstract
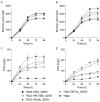
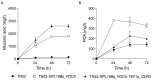
The efficient production of biobased organic acids is crucial to move to a more sustainable and eco-friendly economy, where muconic acid is gaining interest as a versatile platform chemical to produce industrial building blocks, including adipic acid and terephthalic acid. In this study, a Saccharomyces cerevisiae platform strain able to convert glucose and xylose into cis,cis-muconic acid was further engineered to eliminate C2 dependency, improve muconic acid tolerance, enhance production and growth performance, and substantially reduce the side production of the intermediate protocatechuic acid. This was achieved by reintroducing the PDC5 gene and overexpression of QDR3 genes. The improved strain was integrated in low-pH fed-batch fermentations at bioreactor scale with integrated in situ product recovery. By adding a biocompatible organic phase consisting of CYTOP 503 and canola oil to the process, a continuous extraction of muconic acid was achieved, resulting in significant alleviation of product inhibition. Through this, the muconic acid titer and peak productivity were improved by 300% and 185%, respectively, reaching 9.3 g/L and 0.100 g/L/h in the in situ product recovery process as compared to 3.1 g/L and 0.054 g/L/h in the control process without ISPR.
Keywords: Saccharomyces cerevisiae; in situ product recovery; metabolic engineering; muconic acid; reactive extraction; yeast cell factory.
Conflict of interest statement
Authors Sinah Tönjes, Evelien Uitterhaegen, Karel De Winter and Wim Soetaert were employed by the company Bio Base Europe Pilot Plant (BBEPP). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Rorrer N.A., Dorgan J.R., Vardon D.R., Martinez C.R., Yang Y., Beckham G.T. Renewable Unsaturated Polyesters from Muconic Acid. ACS Sustain. Chem. Eng. 2016;4:6867–6876. doi: 10.1021/acssuschemeng.6b01820. - DOI
-
- Zanella E., Secundo L., Bellomi S., Vomeri A., Villa A., Pirola C. Bio-Adipic Acid Production from Muconic Acid Hydrogenation on Palladium-Transition Metal (Ni and Zn) Bimetallic Catalysts. Catalysts. 2023;13:486. doi: 10.3390/catal13030486. - DOI
-
- Rosini E., Molinari F., Miani D., Pollegioni L. Lignin Valorization: Production of High Value-Added Compounds by Engineered Microorganisms. Catalysts. 2023;13:555. doi: 10.3390/catal13030555. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

