Three-Dimensional-Bioprinted Non-Small Cell Lung Cancer Models in a Mouse Phantom for Radiotherapy Research
- PMID: 39408596
- PMCID: PMC11476964
- DOI: 10.3390/ijms251910268
Three-Dimensional-Bioprinted Non-Small Cell Lung Cancer Models in a Mouse Phantom for Radiotherapy Research
Abstract
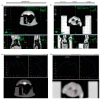
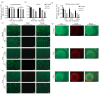
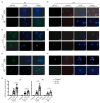
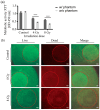
Lung cancer continues to have one of the highest morbidity and mortality rates of any cancer. Although radiochemotherapy, in combination with immunotherapy, has significantly improved overall survival, new treatment options are urgently needed. However, preclinical radiotherapy testing is often performed in animal models, which has several drawbacks, including species-specific differences and ethical concerns. To replace animal models, this study used a micro-extrusion bioprinting approach to generate a three-dimensional (3D) human lung cancer model consisting of lung tumor cells embedded in human primary lung fibroblasts for radiotherapy research. The models were placed in a mouse phantom, i.e., a 3D-printed mouse model made of materials that mimic the X-ray radiation attenuation rates found in mice. In radiotherapy experiments, the model demonstrated a selective cytotoxic effect of X-rays on tumor cells, consistent with findings in 2D cells. Furthermore, the analysis of metabolic activity, cell death, apoptosis, and DNA damage-induced γH2AX foci formation revealed different results in the 3D model inside the phantom compared to those observed in irradiated models without phantom and 2D cells. The proposed setup of the bioprinted 3D lung model inside the mouse phantom provides a physiologically relevant model system to study radiation effects.
Keywords: 3D bioprinting; lung cancer; model system; mouse phantom; radiation therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Meagan M., Nasser H. Advances in systemic therapy for non-small cell lung cancer. BMJ. 2021;375:n2363. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

