Taldefgrobep Alfa and the Phase 3 RESILIENT Trial in Spinal Muscular Atrophy
- PMID: 39408601
- PMCID: PMC11477173
- DOI: 10.3390/ijms251910273
Taldefgrobep Alfa and the Phase 3 RESILIENT Trial in Spinal Muscular Atrophy
Abstract
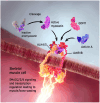
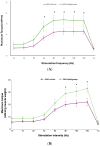
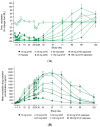
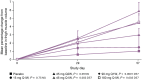
Spinal muscular atrophy (SMA) is a rare, genetic neurodegenerative disorder caused by insufficient production of survival motor neuron (SMN) protein. Diminished SMN protein levels lead to motor neuron loss, causing muscle atrophy and weakness that impairs daily functioning and reduces quality of life. SMN upregulators offer clinical improvements and increased survival in SMA patients, although significant unmet needs remain. Myostatin, a TGF-β superfamily signaling molecule that binds to the activin II receptor, negatively regulates muscle growth; myostatin inhibition is a promising therapeutic strategy for enhancing muscle. Combining myostatin inhibition with SMN upregulation, a comprehensive therapeutic strategy targeting the whole motor unit, offers promise in SMA. Taldefgrobep alfa is a novel, fully human recombinant protein that selectively binds to myostatin and competitively inhibits other ligands that signal through the activin II receptor. Given a robust scientific and clinical rationale and the favorable safety profile of taldefgrobep in patients with neuromuscular disease, the RESILIENT phase 3, randomized, placebo-controlled trial is investigating taldefgrobep as an adjunct to SMN upregulators in SMA (NCT05337553). This manuscript reviews the role of myostatin in muscle, explores the preclinical and clinical development of taldefgrobep and introduces the phase 3 RESILIENT trial of taldefgrobep in SMA.
Keywords: RESILIENT; SMA; SMN upregulation; SMN upregulator; antimyostatin; myostatin; myostatin inhibitor; phase 3 clinical trial; spinal muscular atrophy; taldefgrobep.
Conflict of interest statement
L.S. has served as a consultant for Biogen, Roche, Novartis, Astellas, Pfizer, Sarepta, Evox, PTC, Sysnav, Dyne, Zentech, MitoRx and Biohaven and has also served on a Scientific Advisory or Data Safety Monitoring board for Lupin, Fibrogen, Alltrana, Illumina and Roche. The institution of L.S. has received research support from Roche, Biogen, Zentech, PerkinHalmers, Scholar Rock and Biohaven. L.L.L. is an employee of and has stock in Biohaven. A.M.C. has served on the Scientific Advisory or Data Safety Monitoring board for Edgewise Therapeutics, Octapharma, Sarepta, Avidity, Morphic Therapeutics and Biohaven. B.J.B. has no disclosures to report. K.S.C. has no disclosures to report. V.C. is an employee and member of the Board of Directors of and has stock in Biohaven. I.Q. is an employee of and has stock in Biohaven. S.D. is an employee of and has stock in Biohaven. D.J.C. is an employee of and has stock in Biohaven. G.M. is a past employee of Biohaven. J.M. is an employee of and has stock in Biohaven. C.B. has stock in Biohaven and is a member of the Board of Directors of Biohaven Biosciences Ireland Limited.
Figures




References
-
- Burr P., Reddivari A.K.R. National Library of Medicine. [(accessed on 14 May 2024)]; Available online: http://www.ncbi.nlm.nih.gov/books/NBK560687/
-
- Calucho M., Bernal S., Alías L., March F., Venceslá A., Rodríguez-Álvarez F.J., Aller E., Fernández R.M., Borrego S., Millán J.M., et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018;28:208–215. doi: 10.1016/j.nmd.2018.01.003. - DOI - PubMed
-
- Lair L., Qureshi I., Bechtold C., Heller L., Durham S., Campbell D., Marin J., Chen K., Coric V. P04 Taldefgrobep alfa: Preclinical and clinical data supporting the phase 3 RESILIENT study in spinal muscular atrophy. Neuromuscul. Disord. 2023;33:S163. doi: 10.1016/j.nmd.2023.07.381. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

