New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol
- PMID: 39408621
- PMCID: PMC11477119
- DOI: 10.3390/ijms251910291
New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol
Abstract
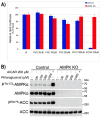
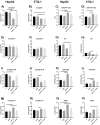
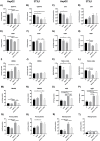
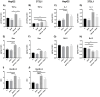
Phloroglucinol (PHG), an analgesic and spasmolytic drug, shows promise in preventing high-fat-diet (HFD)-induced non-alcoholic fatty liver disease (NAFLD) and insulin resistance. In Wistar rats, 10 weeks of PHG treatment did not prevent HFD-induced weight gain but significantly mitigated fasting hyperglycemia, impaired insulin responses, and liver steatosis. This protective effect was not linked to hepatic lipogenesis or AMP-activated protein kinase (AMPK) activation. Instead, PHG improved mitochondrial function by reducing oxidative stress, enhancing ATP production, and increasing anti-oxidant enzyme activity. PHG also relaxed gastric smooth muscles via potassium channel activation and nitric oxide (NO) signaling, potentially delaying gastric emptying. A pilot intervention in pre-diabetic men confirmed PHG's efficacy in improving postprandial glycemic control and altering lipid metabolism. These findings suggest PHG as a potential therapeutic for NAFLD and insulin resistance, acting through mechanisms involving mitochondrial protection, anti-oxidant activity, and gastric motility modulation. Further clinical evaluation is warranted to explore PHG's full therapeutic potential.
Keywords: NAFLD; anti-spasmodic; diabetes; insulin resistance; lipid metabolism; liver steatosis; oxidative stress; phloroglucinol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Chavin K.D., Yang S.Q., Lin H.Z., Chatham J., Chacko V.P., Hock J.B., Walajtys-Rode E., Rashid A., Chen C.H., Huang C.C., et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J. Biol. Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

