Blockade of mTORC1 via Rapamycin Suppresses 27-Hydroxycholestrol-Induced Inflammatory Responses
- PMID: 39408711
- PMCID: PMC11477202
- DOI: 10.3390/ijms251910381
Blockade of mTORC1 via Rapamycin Suppresses 27-Hydroxycholestrol-Induced Inflammatory Responses
Abstract
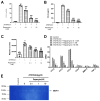
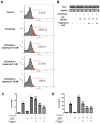
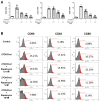
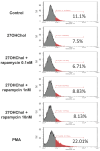
Atherosclerosis is characterized by the deposition and accumulation of extracellular cholesterol and inflammatory cells in the arterial blood vessel walls, and 27-hydroxycholesterol (27OHChol) is the most abundant cholesterol metabolite. 27OHChol is an oxysterol that induces immune responses, including immune cell activation and chemokine secretion, although the underlying mechanisms are not fully understood. In this study, we investigated the roles of the mechanistic target of rapamycin (mTOR) in 27HChol-induced inflammation using rapamycin. Treating monocytic cells with rapamycin effectively reduced the expression of CCL2 and CD14, which was involved with the increased immune response by 27OHChol. Rapamycin also suppressed the phosphorylation of S6 and 4EBP1, which are downstream of mTORC1. Additionally, it also alleviates the increase in differentiation markers into macrophage. These results suggest that 27OHChol induces inflammation by activating the mTORC1 signaling pathway, and rapamycin may be useful for the treatment of atherosclerosis-related inflammation involving 27OHchol.
Keywords: 27-hydroxycholesterol; inflammation; mTOR; monocytic cells; rapamycin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Berthier A., Bessède G., Corcos L., Gambert P., Néel D., Lizard G. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 2005;21:97–114. doi: 10.1007/s10565-005-0141-2. - DOI - PubMed
-
- Iuliano L., Micheletta F., Natoli S., Ginanni Corradini S., Iappelli M., Elisei W., Giovannelli L., Violi F., Diczfalusy U. Measurement of oxysterols and alpha-tocopherol in plasma and tissue samples as indices of oxidant stress status. Anal. Biochem. 2003;312:217–223. doi: 10.1016/S0003-2697(02)00467-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

