Telomere Reprogramming and Cellular Metabolism: Is There a Link?
- PMID: 39408829
- PMCID: PMC11476947
- DOI: 10.3390/ijms251910500
Telomere Reprogramming and Cellular Metabolism: Is There a Link?
Abstract
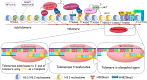
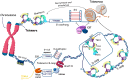
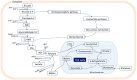
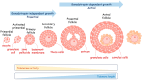
Telomeres-special DNA-protein structures at the ends of linear eukaryotic chromosomes-define the proliferation potential of cells. Extremely short telomeres promote a DNA damage response and cell death to eliminate cells that may have accumulated mutations after multiple divisions. However, telomere elongation is associated with the increased proliferative potential of specific cell types, such as stem and germ cells. This elongation can be permanent in these cells and is activated temporally during immune response activation and regeneration processes. The activation of telomere lengthening mechanisms is coupled with increased proliferation and the cells' need for energy and building resources. To obtain the necessary nutrients, cells are capable of finely regulating energy production and consumption, switching between catabolic and anabolic processes. In this review, we focused on the interconnection between metabolism programs and telomere lengthening mechanisms during programmed activation of proliferation, such as in germ cell maturation, early embryonic development, neoplastic lesion growth, and immune response activation. It is generally accepted that telomere disturbance influences biological processes and promotes dysfunctionality. Here, we propose that metabolic conditions within proliferating cells should be involved in regulating telomere lengthening mechanisms, and telomere length may serve as a marker of defects in cellular functionality. We propose that it is possible to reprogram metabolism in order to regulate the telomere length and proliferative activity of cells, which may be important for the development of approaches to regeneration, immune response modulation, and cancer therapy. However, further investigations in this area are necessary to improve the understanding and manipulation of the molecular mechanisms involved in the regulation of proliferation, metabolism, and aging.
Keywords: ALT; OXPHOS; alternative lengthening of telomeres; development; glycolysis; metabolism; oxidative phosphorylation; telomerase; telomere.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Fumagalli M., Rossiello F., Clerici M., Barozzi S., Cittaro D., Kaplunov J.M., Bucci G., Dobreva M., Matti V., Beausejour C.M., et al. Telomeric DNA Damage Is Irreparable and Causes Persistent DNA-Damage-Response Activation. Nat. Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

