Interactions between Brassinosteroids and Strigolactones in Alleviating Salt Stress in Maize
- PMID: 39408841
- PMCID: PMC11477198
- DOI: 10.3390/ijms251910505
Interactions between Brassinosteroids and Strigolactones in Alleviating Salt Stress in Maize
Abstract
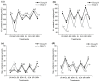
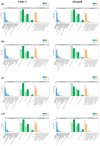
Exogenous brassinolide (BR) and strigolactones (SLs) play an important role in alleviating salt stress in maize. We studied the morphological and physiological responses of the salt-sensitive genotype PH4CV and salt-tolerant genotype Zheng58 to BR (1.65 nM), SL (1 µM), and BS (1.65 nM BR + 1 µM SL) under salt stress. Phenotypic analysis showed that salt stress significantly inhibited the growth of maize seedlings and significantly increased the content of Na+ in the roots. Exogenous hormones increased oxidase activity and decreased Na+ content in the roots and mitigated salt stress. Transcriptome analysis showed that the interaction of BR and SL is involved in photosynthesis-antenna proteins, the TCA cycle, and plant hormone signal transduction pathways. This interaction influences the expression of chlorophyll a/b-binding protein and glucose-6-phosphate isomerase 1 chloroplastic, and aconitase genes are affected. Furthermore, the application of exogenous hormones regulates the expression of genes associated with the signaling pathways of cytokinin (CK), gibberellins (GA), auxin (IAA), brassinosteroid (BR), abscisic acid (ABA), and jasmonic acid (JA). Additionally, exogenous hormones inhibit the expression of the AKT2/3 genes, which are responsible for regulating ion transduction and potassium ion influx. Four candidate genes that may regulate the seedling length of maize were screened out through WGCNA. Respective KOG notes concerned inorganic ion transport and metabolism, signal transduction mechanisms, energy production and conversion, and amino acid transport and metabolism. The findings of this study provide a foundation for the proposition that BR and SL can be employed to regulate salt stress alleviation in maize.
Keywords: brassinosteroids; hormone interactions; maize (Zea mays L.); salt stress; strigolactones.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
MeSH terms
Substances
Grants and funding
- 202401036、202401046、202401035/the College Students' Innovation and Entrepreneurship Training Program of Gansu Agricultural University
- 23ZYQA0322/Central Guide Local Science and Technology Development Fund Project
- 22ZD6NA009/Gansu Province Science and Technology Plan-Major Project
- 2022YFD1201804/National Key Research and Development Project
- 2022CYZC-46/Gansu Province Higher Education Industry Support Plan
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

