Nature of Charge Transfer Effects in Complexes of Dopamine Derivatives Adsorbed on Graphene-Type Nanostructures
- PMID: 39408851
- PMCID: PMC11477014
- DOI: 10.3390/ijms251910522
Nature of Charge Transfer Effects in Complexes of Dopamine Derivatives Adsorbed on Graphene-Type Nanostructures
Abstract
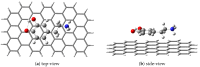
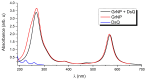
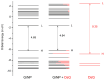
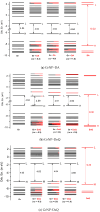
Continuing the investigation started for dopamine (DA) and dopamine-o-quinone (DoQ) (see, the light absorption and charge transfer properties of the dopamine zwitterion (called dopamine-semiquinone or DsQ) adsorbed on the graphene nanoparticle surface is investigated using the ground state and linear-response time-dependent density functional theories, considering the ωB97X-D3BJ/def2-TZVPP level of theory. In terms of the strength of molecular adsorption on the surface, the DsQ form has 50% higher binding energy than that found in our previous work for the DA or DoQ cases (-20.24 kcal/mol vs. -30.41 kcal/mol). The results obtained for electronically excited states and UV-Vis absorption spectra show that the photochemical behavior of DsQ is more similar to DA than that observed for DoQ. Of the three systems analyzed, the DsQ-based complex shows the most active charge transfer (CT) phenomenon, both in terms of the number of CT-like states and the amount of charge transferred. Of the first thirty electronically excited states computed for the DsQ case, eleven are purely of the CT type, and nine are mixed CT and localized (or Frenkel) excitations. By varying the adsorption distance between the molecule and the surface vertically, the amount of charge transfer obtained for DA decreases significantly as the distance increases: for DoQ it remains stable, for DsQ there are states for which little change is observed, and for others, there is a significant change. Furthermore, the mechanistic compilation of the electron orbital diagrams of the individual components cannot describe in detail the nature of the excitations inside the complex.
Keywords: TDDFT; charge transfer; dopamine; graphene; quinone; zwitterion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Theoretical insights into dopamine photochemistry adsorbed on graphene-type nanostructures.Phys Chem Chem Phys. 2024 May 22;26(20):14937-14947. doi: 10.1039/d4cp00432a. Phys Chem Chem Phys. 2024. PMID: 38738904
-
Atomistic Simulations of Dopamine Diffusion Dynamics on a Pristine Graphene Surface.Chemphyschem. 2022 Feb 16;23(4):e202100783. doi: 10.1002/cphc.202100783. Epub 2022 Jan 20. Chemphyschem. 2022. PMID: 34939307 Free PMC article.
-
Noncovalent Interactions between Dopamine and Regular and Defective Graphene.Chemphyschem. 2017 Aug 5;18(15):2065-2080. doi: 10.1002/cphc.201700252. Epub 2017 Jun 8. Chemphyschem. 2017. PMID: 28494119
-
Competitive adsorption of dopamine and rhodamine 6G on the surface of graphene oxide.ACS Appl Mater Interfaces. 2014 Feb 26;6(4):2459-70. doi: 10.1021/am404881p. Epub 2014 Feb 4. ACS Appl Mater Interfaces. 2014. PMID: 24494630
-
Novel behavior of monolayer quantum gases on graphene, graphane and fluorographene.J Phys Condens Matter. 2013 Nov 6;25(44):443001. doi: 10.1088/0953-8984/25/44/443001. Epub 2013 Oct 10. J Phys Condens Matter. 2013. PMID: 24113280 Review.
References
-
- Bakulin A.A., Dimitrov S.D., Rao A., Chow P.C.Y., Nielsen C.B., Schroeder B.C., McCulloch I., Bakker H.J., Durrant J.R., Friend R.H. Charge-Transfer State Dynamics Following Hole and Electron Transfer in Organic Photovoltaic Devices. J. Phys. Chem. Lett. 2013;4:209–215. doi: 10.1021/jz301883y. - DOI - PubMed
-
- Holliday S., Li Y., Luscombe C.K. Recent advances in high performance donor-acceptor polymers for organic photovoltaics. Prog. Polym. Sci. 2017;70:34–51. doi: 10.1016/j.progpolymsci.2017.03.003. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

