IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes
- PMID: 39408857
- PMCID: PMC11476353
- DOI: 10.3390/ijms251910530
IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes
Abstract
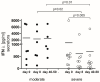
Interferons (IFNs) produced by airway epithelial cells are crucial in defending against pathogens. Fluctuations in IFN-λ levels may influence coronavirus disease 19 (COVID-19) severity. However, conflicting data have been reported regarding serum IFN-λ concentrations in COVID-19 patients. To address this, we evaluated serum IFN-λ levels over time in moderate and severe COVID-19 patients and their association with cytokine production and clinical parameters using the enzyme-linked immunosorbent assay (ELISA) and the Bio-Plex Pro Human Cytokine 17-plex Assay. Results from testing 51 COVID-19 patients showed that 68% lacked detectable serum IFN-λ. Among non-IFN-λ secretors, severe COVID-19 predominated. In contrast, IFN-λ secretors displayed stable IFN-λ levels in moderate cases, while severe cases showed a decline over time, which persisted even after recovery. A negative correlation was observed between IFN-λ levels and inflammatory markers. This, combined with an increase in tumor necrosis factor alpha (TNF-α) and clinical improvement, suggests a regulatory role for IFN-λ in promoting faster recovery. Despite this, survival rates were similar between the groups, indicating that while IFN-λ influences the course of the disease, it does not directly affect overall survival. In conclusion, IFN-λ is vital, but not unique, for the antiviral response and COVID-19 recovery. Simultaneously, serum IFN-λ deficiency signifies severe COVID-19.
Keywords: COVID-19; SARS-CoV-2; innate immunity; interferon.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Linkage of Lambda Interferons in Protection Against Severe COVID-19.J Interferon Cytokine Res. 2021 Apr;41(4):149-152. doi: 10.1089/jir.2020.0187. J Interferon Cytokine Res. 2021. PMID: 33885337 Clinical Trial.
-
Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison.Nat Immunol. 2021 Jan;22(1):32-40. doi: 10.1038/s41590-020-00840-x. Epub 2020 Dec 4. Nat Immunol. 2021. PMID: 33277638
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Type I and III interferon responses in SARS-CoV-2 infection.Exp Mol Med. 2021 May;53(5):750-760. doi: 10.1038/s12276-021-00592-0. Epub 2021 May 6. Exp Mol Med. 2021. PMID: 33953323 Free PMC article. Review.
-
Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection.Eur J Immunol. 2021 May;51(5):1071-1075. doi: 10.1002/eji.202149173. Epub 2021 Mar 22. Eur J Immunol. 2021. PMID: 33675065 Free PMC article. Review.
Cited by
-
Associations between type III interferons, obesity and clinical severity of COVID-19.Front Immunol. 2025 Apr 22;16:1516756. doi: 10.3389/fimmu.2025.1516756. eCollection 2025. Front Immunol. 2025. PMID: 40330483 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

