What Do We Know about Peripartum Cardiomyopathy? Yesterday, Today, Tomorrow
- PMID: 39408885
- PMCID: PMC11477285
- DOI: 10.3390/ijms251910559
What Do We Know about Peripartum Cardiomyopathy? Yesterday, Today, Tomorrow
Abstract
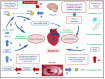
Peripartum cardiomyopathy is a disease that occurs during or after pregnancy and leads to a significant decline in cardiac function in previously healthy women. Peripartum cardiomyopathy has a varying prevalence among women depending on the part of the world where they live, but it is associated with a significant mortality and morbidity in this population. Therefore, timely diagnosis, treatment, and monitoring of this disease from its onset are of utmost importance. Although many risk factors are associated with the occurrence of peripartum cardiomyopathy, such as conditions of life, age of the woman, nutrient deficiencies, or multiple pregnancies, the exact cause of its onset remains unknown. Advances in research on the genetic associations with cardiomyopathies have provided a wealth of data indicating a possible association with peripartum cardiomyopathy, but due to numerous mutations and data inconsistencies, the exact connection remains unclear. Significant insights into the pathophysiological mechanisms underlying peripartum cardiomyopathy have been provided by the theory of an abnormal 16-kDa prolactin, which may be generated in an oxidative stress environment and lead to vascular and consequently myocardial damage. Recent studies supporting this disease mechanism also include research on the efficacy of bromocriptine (a prolactin synthesis inhibitor) in restoring cardiac function in affected patients. Despite significant progress in the research of this disease, there are still insufficient data on the safety of use of certain drugs treating heart failure during pregnancy and breastfeeding. Considering the metabolic changes that occur in different stages of pregnancy and the postpartum period, determining the correct dosing regimen of medications is of utmost importance not only for better treatment and survival of mothers but also for reducing the risk of toxic effects on the fetus.
Keywords: heart failure; peripartum cardiomyopathy; pregnancy; prognosis; treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Hull E., Hafkesbring E. Toxic Postpartal Heart Disease. New Orleans Med. Surg. J. 1937;89:550–557.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

