TongGuanWan Alleviates Doxorubicin- and Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis by Modulating Apoptotic and Fibrotic Pathways
- PMID: 39408900
- PMCID: PMC11476530
- DOI: 10.3390/ijms251910573
TongGuanWan Alleviates Doxorubicin- and Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis by Modulating Apoptotic and Fibrotic Pathways
Abstract
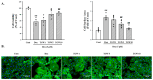
Heart failure, a major public health issue, often stems from prolonged stress or damage to the heart muscle, leading to cardiac hypertrophy. This can progress to heart failure and other cardiovascular problems. Doxorubicin (DOX), a common chemotherapy drug, and isoproterenol (ISO), a β-adrenergic agonist, both induce cardiac hypertrophy through different mechanisms. This study investigates TongGuanWan (TGW,), a traditional herbal remedy, for its effects on cardiac hypertrophy and fibrosis in DOX-induced H9c2 cells and ISO-induced mouse models. TGW was found to counteract DOX-induced increases in H9c2 cell surface area (n = 8, p < 0.01) and improve biomarkers like ANP (n = 3, p < 0.01)) and BNP (n = 3, p < 0.01). It inhibited the MAPK pathway (n = 4, p < 0.01) and GATA-4/calcineurin/NFAT-3 signaling, reduced inflammation by decreasing NF-κB p65 translocation, and enhanced apoptosis-related factors such as caspase-3 (n = 3, p < 0.01), caspase-9 (n = 3, p < 0.01), Bax (n = 3, p < 0.01), and Bcl-2 (n = 3, p < 0.01). Flow cytometry showed TGW reduced apoptotic cell populations. In vivo, TGW reduced heart (n = 8~10, p < 0.01), and left ventricle weights (n = 6~7), cardiac hypertrophy markers (n = 3, p < 0.01), and perivascular fibrosis in ISO-induced mice, with Western blot analysis confirming decreased levels of fibrosis-related factors like fibronectin, α-SMA (n = 3, p < 0.05), and collagen type I (n = 3, p < 0.05). These findings suggest TGW has potential as a therapeutic option for cardiac hypertrophy and fibrosis.
Keywords: TongGuanWan; apoptosis; cardiac hypertrophy; doxorubicin; fibrosis; heart failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Pallazola V.A., Davis D.M., Whelton S.P., Cardoso R., Latina J.M., Michos E.D., Sarkar S., Blumenthal R.S., Arnett D.K., Stone N.J., et al. A clinician’s guide to healthy eating for cardiovascular disease prevention. Mayo Clin. Proc. Innov. Qual. Outcomes. 2019;3:251–267. doi: 10.1016/j.mayocpiqo.2019.05.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

