3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds
- PMID: 39408911
- PMCID: PMC11477194
- DOI: 10.3390/ijms251910582
3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds
Abstract
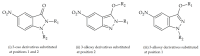
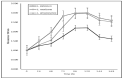
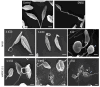
Indazoles have previously been identified as molecules with antiprotozoal activity. In this study, we evaluate the in vitro activity of thirteen 3-alkoxy-1-benzyl-5-nitroindazole derivatives (series D) against L. amazonensis, L. infantum, and L. mexicana. In vitro, cytotoxicity against mouse peritoneal macrophages and growth inhibitory activity in promastigotes were evaluated for all compounds, and those showing adequate activity and selectivity were tested against intracellular amastigotes. Transmission and scanning electron microscopy were employed to study the effects of 3-alkoxy-1-benzyl-5-nitroindazole and 2-benzyl-5-nitroindazolin-3-one derivatives on promastigotes of L. amazonensis. Compounds NV6 and NV8 were active in the two life stages of the three species, with the latter showing the best indicators of activity and selectivity. 3-alkoxy-1-benzyl-5-nitroindazole derivatives (series D) showed in vitro activity comparable to that of amphotericin B against the promastigote stage of Leishmania spp. Two compounds were also found to be active the amastigote stage. Electron microscopy studies confirmed the antileishmanial activity of the indazole derivatives studied and support future research on this family of compounds as antileishmanial agents.
Keywords: 2-benzyl-5-nitroindazolin-3-one; 3-alkoxy-1-benzyl-5-nitroindazole; amastigote; antileishmanial activity; leishmaniasis; promastigote.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
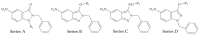


Similar articles
-
Antileishmanial activity of 5-nitroindazole derivatives.Ther Adv Infect Dis. 2023 Oct 30;10:20499361231208294. doi: 10.1177/20499361231208294. eCollection 2023 Jan-Dec. Ther Adv Infect Dis. 2023. PMID: 37915499 Free PMC article.
-
Antileishmanial compounds from Connarus suberosus: Metabolomics, isolation and mechanism of action.PLoS One. 2020 Nov 6;15(11):e0241855. doi: 10.1371/journal.pone.0241855. eCollection 2020. PLoS One. 2020. PMID: 33156835 Free PMC article.
-
In Vitro and In Vivo Antileishmanial Activity of Thioridazine.Acta Parasitol. 2024 Mar;69(1):324-331. doi: 10.1007/s11686-023-00746-2. Epub 2023 Dec 9. Acta Parasitol. 2024. PMID: 38070122 Free PMC article.
-
Antileishmanial Activities of Medicinal Herbs and Phytochemicals In Vitro and In Vivo: An Update for the Years 2015 to 2021.Molecules. 2022 Nov 4;27(21):7579. doi: 10.3390/molecules27217579. Molecules. 2022. PMID: 36364404 Free PMC article. Review.
-
Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c.Nitric Oxide. 2016 Aug 31;58:51-8. doi: 10.1016/j.niox.2016.06.004. Epub 2016 Jun 18. Nitric Oxide. 2016. PMID: 27328771 Review.
References
-
- Schmidt A. Advances in Heterocyclic Chemistry. Volume 85. Elsevier; Amsterdam, The Netherlands: 2003. [(accessed on 5 April 2024)]. p. 67. Available online: https://shop.elsevier.com/books/advances-in-heterocyclic-chemistry/katri....
-
- Behrouz S. Highly efficient one-pot three component synthesis of 2H-indazoles by consecutive condensation, C-N and N-N bond for-mations using Cu/Aminoclay/reduced grapheme oxide nanohybrid. J. Heterocycl. Chem. 2016;54:1863–1871. doi: 10.1002/jhet.2777. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

