Imaging the Raf-MEK-ERK Signaling Cascade in Living Cells
- PMID: 39408915
- PMCID: PMC11477372
- DOI: 10.3390/ijms251910587
Imaging the Raf-MEK-ERK Signaling Cascade in Living Cells
Abstract
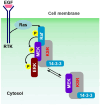
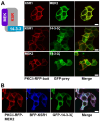
Conventional biochemical methods for studying cellular signaling cascades have relied on destructive cell disruption. In contrast, the live cell imaging of fluorescent-tagged transfected proteins offers a non-invasive approach to understanding signal transduction events. One strategy involves monitoring the phosphorylation-dependent shuttling of a fluorescent-labeled kinase between the nucleus and cytoplasm using nuclear localization, export signals, or both. In this paper, we introduce a simple method to visualize intracellular signal transduction in live cells by exploring the translocation properties of PKC from the cytoplasm to the membrane. We fused bait protein to PKC, allowing the bait (RFP-labeled) and target (GFP-labeled) proteins to co-translocate from the cytoplasm to the membrane. However, in non-interacting protein pairs, only the bait protein was translocated to the plasma membrane. To verify our approach, we examined the Raf-MEK-ERK signaling cascade (ERK pathway). We successfully visualized direct Raf1/MEK2 interaction and the KSR1-containing ternary complex (Raf1/MEK2/KSR1). However, the interaction between MEK and ERK was dependent on the presence of the KSR1 scaffold protein under our experimental conditions.
Keywords: ERK pathway; Raf–MEK–ERK signaling cascade; cell-based assay; scaffold protein; visualizing protein interaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

