The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema
- PMID: 39408941
- PMCID: PMC11477147
- DOI: 10.3390/ijms251910613
The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema
Abstract
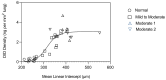
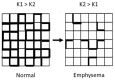
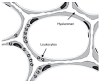
Pulmonary emphysema involves progressive destruction of alveolar walls, leading to enlarged air spaces and impaired gas exchange. While the precise mechanisms responsible for these changes remain unclear, there is growing evidence that the extracellular matrix plays a critical role in the process. An essential feature of pulmonary emphysema is damage to the elastic fiber network surrounding the airspaces, which stores the energy needed to expel air from the lungs. The degradation of these fibers disrupts the mechanical forces involved in respiration, resulting in distension and rupture of alveolar walls. While the initial repair process mainly consists of elastin degradation and resynthesis, continued alveolar wall injury may be associated with increased collagen deposition, resulting in a mixed pattern of emphysema and interstitial fibrosis. Due to the critical role of elastic fiber injury in pulmonary emphysema, preventing damage to this matrix component has emerged as a potential therapeutic strategy. One treatment approach involves the intratracheal administration of hyaluronan, a polysaccharide that prevents elastin breakdown by binding to lung elastic fibers. In clinical trials, inhalation of aerosolized HA decreased elastic fiber injury, as measured by the release of the elastin-specific cross-linking amino acids, desmosine, and isodesmosine. By protecting elastic fibers from enzymatic and oxidative damage, aerosolized HA could alter the natural history of pulmonary emphysema, thereby reducing the risk of respiratory failure.
Keywords: collagen; elastin; extracellular matrix; hyaluronan; pulmonary emphysema.
Conflict of interest statement
Jerome Cantor is an inventor on USPTO patent number 10933084 entitled “Compositions and Methods for Treating Elastic Fiber Breakdown”.
Figures

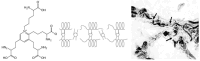


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

