Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury
- PMID: 39408943
- PMCID: PMC11477367
- DOI: 10.3390/ijms251910615
Hydrogel-Mediated Local Delivery of Induced Nephron Progenitor Cell-Sourced Molecules as a Cell-Free Approach for Acute Kidney Injury
Abstract
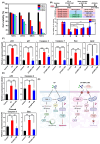
Acute kidney injury (AKI) constitutes a severe condition characterized by a sudden decrease in kidney function. Utilizing lineage-restricted stem/progenitor cells, directly reprogrammed from somatic cells, is a promising therapeutic option in personalized medicine for serious and incurable diseases such as AKI. The present study describes the therapeutic potential of induced nephron progenitor cell-sourced molecules (iNPC-SMs) as a cell-free strategy against cisplatin (CP)-induced nephrotoxicity, employing hyaluronic acid (HA) hydrogel-mediated local delivery to minimize systemic leakage and degradation. iNPC-SMs exhibited anti-apoptotic effects on HK-2 cells by inhibiting CP-induced ROS generation. Additionally, the localized biodistribution facilitated by hydrogel-mediated iNPC-SM delivery contributed to enhanced renal function, anti-inflammatory response, and renal regeneration in AKI mice. This study could serve as a 'proof of concept' for injectable hydrogel-mediated iNPC-SM delivery in AKI and as a model for further exploration of the development of cell-free regenerative medicine strategies.
Keywords: cell-free regenerative medicine; induced nephron progenitor cells; injectable hydrogel.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

