Unveiling the Role of Two Rhodopsin-like GPCR Genes in Insecticide-Resistant House Flies, Musca domestica
- PMID: 39408947
- PMCID: PMC11477390
- DOI: 10.3390/ijms251910618
Unveiling the Role of Two Rhodopsin-like GPCR Genes in Insecticide-Resistant House Flies, Musca domestica
Abstract
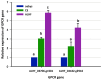
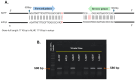
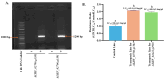
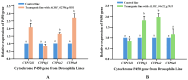
Insecticide resistance in insects, driven by the overexpression of P450 enzymes, presents a significant challenge due to the enhanced metabolic detoxification of insecticides. Although the transcriptional regulation of P450 genes is not yet fully understood, G-protein-coupled receptor (GPCR) genes have emerged as key regulators in this process. This study is the first to associate GPCR genes with insecticide resistance in Musca domestica. We identified two key rhodopsin-like GPCR genes, ALHF_02706.g1581 and ALHF_04422.g2918, which were significantly overexpressed in the resistant ALHF strain compared to sensitive strains. Notably, both ALHF_02706.g1581 and ALHF_04422.g2918 were mapped to autosome 2, where critical but unidentified regulatory factors controlling resistance and P450 gene regulation are located. This supports our hypothesis that GPCRs function as trans-regulatory factors for P450-mediated resistance. Functional analysis using transgenic Drosophila demonstrated that overexpression of these rhodopsin-like GPCR genes increased permethrin resistance by approximately two-fold. Specifically, ALHF_02706.g1581 overexpression significantly upregulated the Drosophila resistance-related P450 genes CYP12D1, CYP6A2, and CYP6A8, while ALHF_04422.g2918 increased CYP6G1 and CYP6A2 expression, thereby enhancing insecticide detoxification in rhodopsin-like GPCR transgenic Drosophila lines. These findings suggest that these rhodopsin-like GPCR genes on autosome 2 may act as trans-regulatory factors for P450-mediated resistance, underscoring their critical role in insecticide detoxification and resistance development in M. domestica.
Keywords: G-protein coupled receptors (GPCRs); cytochrome P450s; gene mapping; house flies; insecticide resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Geden C.J., Nayduch D., Scott J.G., Burgess E.R., Gerry A.C., Kaufman P.E., Thomson J., Pickens V., Machtinger E.T. House fly (Diptera: Muscidae): Biology, pest status, current management prospects, and research needs. J. Integr. Pest. Manag. 2021;12:39. doi: 10.1093/jipm/pmaa021. - DOI
-
- Nayduch D., Burrus R.G. Flourishing in filth: House fly–microbe interactions across life history. Ann. Entomol. Soc. Am. 2017;110:6–18. doi: 10.1093/aesa/saw083. - DOI
-
- Liu N., Yue X. Genetics of pyrethroid resistance in a strain (ALHF) of house flies (diptera: Muscidae) Pestic. Biochem. Phys. 2001;70:151–158. doi: 10.1006/pest.2001.2547. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

