Intrathecal Immunoselective Nanopheresis for Alzheimer's Disease: What and How? Why and When?
- PMID: 39408961
- PMCID: PMC11476806
- DOI: 10.3390/ijms251910632
Intrathecal Immunoselective Nanopheresis for Alzheimer's Disease: What and How? Why and When?
Abstract
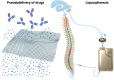
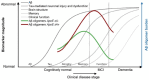
Nanotechnology is transforming therapeutics for brain disorders, especially in developing drug delivery systems. Intrathecal immunoselective nanopheresis with soluble monoclonal antibodies represents an innovative approach in the realm of drug delivery systems for Central Nervous System conditions, especially for targeting soluble beta-amyloid in Alzheimer's disease. This review delves into the concept of intrathecal immunoselective nanopheresis. It provides an overall description of devices to perform this technique while discussing the nanotechnology behind its mechanism of action, its potential advantages, and clinical implications. By exploring current research and advancements, we aim to provide a comprehensive understanding of this novel method, addressing the critical questions of what it is, how it works, why it is needed, and when it should be applied. Special attention is given to patient selection and the optimal timing for therapy initiation in Alzheimer's, coinciding with the peak accumulation of amyloid oligomers in the early stages. Potential limitations and alternative targets beyond beta-amyloid and future perspectives for immunoselective nanopheresis are also described.
Keywords: Alzheimer’s disease; amyloid-oligomers; drug delivery systems; immunoselective nanopheresis; intrathecal delivery; monoclonal antibodies; nanoporous membranes; neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. - DOI - PMC - PubMed
-
- Bors L.A., Erdő F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019;87:6. doi: 10.3390/scipharm87010006. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

