Inhibitory Effects of Reynoutria japonica Houtt. on Pain and Cartilage Breakdown in Osteoarthritis Based on Its Multifaceted Anti-Inflammatory Activity: An In Vivo and In Vitro Approach
- PMID: 39408977
- PMCID: PMC11476456
- DOI: 10.3390/ijms251910647
Inhibitory Effects of Reynoutria japonica Houtt. on Pain and Cartilage Breakdown in Osteoarthritis Based on Its Multifaceted Anti-Inflammatory Activity: An In Vivo and In Vitro Approach
Abstract
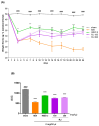
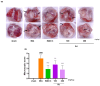
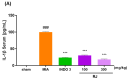
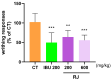
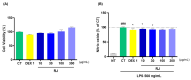
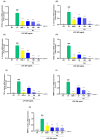
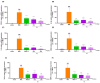
In the past 30 years, the number of years lived with disability due to osteoarthritis (OA) has doubled, making it an increasing global health burden. To address this issue, interventions that inhibit the progressive pathology driven by age-related low-grade inflammation, the primary mechanism of OA, are being actively pursued. Recent investigations have focused on modulating the age-related low-grade inflammatory pathology of this disease as a therapeutic target. However, no agent has successfully halted the disease's progression or reversed its irreversible course. Reynoutria japonica Houtt. (RJ), a promising East Asian herbal medicine, has been utilized for several diseases due to its potent anti-inflammatory activity. This study aims to determine RJ's capacity to inhibit OA symptoms and associated inflammation, exploring its potential for further development. In vivo and in vitro experiments demonstrated RJ's anti-OA activity and modulation of multifaceted inflammatory targets. RJ significantly inhibited pain, gait deterioration, and cartilage destruction in a monosodium iodoacetate-induced OA rat model, with its analgesic effect further confirmed in an acetic acid-induced writhing model. RJ exhibited consistent anti-inflammatory activity against multiple targets in serum and cartilage of the OA rat model and lipopolysaccharide-induced RAW 264.7 cells. The inhibition of inflammatory cytokines, including interleukin-1β, interleukin-6, matrix metalloproteinase-13, tumor necrosis factor-α, and nitric oxide synthase 2, suggests that RJ's alleviation of OA manifestations relates to its multifaceted anti-inflammatory activity. These results indicate that RJ merits further investigation as a disease-modifying drug candidate targeting OA's inflammatory pathology. To further characterize the pharmacological properties of RJ, future studies with expanded designs are warranted.
Keywords: East Asian herbal medicine; Reynoutria japonica Houtt.; analgesic; anti-inflammatory; chondroprotective; osteoarthritis.
Conflict of interest statement
Author Hee-Geun Jo was employed by the company Naturalis Inc. Author Eunhye Baek was employed by the company RexSoft Inc. The all authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. In addition, there is no significant financial support affecting the outcomes of this study.
Figures






Similar articles
-
Pain Relief, Functional Recovery, and Chondroprotective Effects of Angelica gigas Nakai in Osteoarthritis Due to Its Anti-Inflammatory Property: An In Vitro and In Vivo Study.Nutrients. 2024 Jul 26;16(15):2435. doi: 10.3390/nu16152435. Nutrients. 2024. PMID: 39125316 Free PMC article.
-
Investigating the Anti-Inflammatory, Analgesic, and Chondroprotective Effects of Gynostemma pentaphyllum (Thunb.) Makino in Osteoarthritis: An In Vitro and In Vivo Study.Int J Mol Sci. 2024 Sep 4;25(17):9594. doi: 10.3390/ijms25179594. Int J Mol Sci. 2024. PMID: 39273553 Free PMC article.
-
Anti-Inflammatory, Analgesic, Functional Improvement, and Chondroprotective Effects of Erigeron breviscapus (Vant.) Hand.-Mazz. Extract in Osteoarthritis: An In Vivo and In Vitro Study.Nutrients. 2024 Apr 2;16(7):1035. doi: 10.3390/nu16071035. Nutrients. 2024. PMID: 38613068 Free PMC article.
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
-
Developing anti-inflammatory therapeutics for patients with osteoarthritis.Rheumatology (Oxford). 2017 Jun 1;56(6):869-881. doi: 10.1093/rheumatology/kew278. Rheumatology (Oxford). 2017. PMID: 27498352 Review.
Cited by
-
Trifolirhizin: A Phytochemical with Multiple Pharmacological Properties.Molecules. 2025 Jan 17;30(2):383. doi: 10.3390/molecules30020383. Molecules. 2025. PMID: 39860257 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

