Exploring Lysophosphatidylcholine as a Biomarker in Ischemic Stroke: The Plasma-Brain Disjunction
- PMID: 39408978
- PMCID: PMC11477326
- DOI: 10.3390/ijms251910649
Exploring Lysophosphatidylcholine as a Biomarker in Ischemic Stroke: The Plasma-Brain Disjunction
Abstract
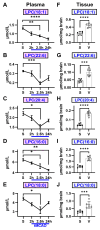
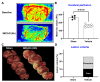
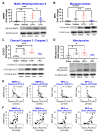
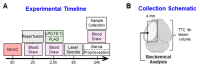
Lipids and their bioactive metabolites, notably lysophosphatidylcholine (LPC), are increasingly important in ischemic stroke research. Reduced plasma LPC levels have been linked to stroke occurrence and poor outcomes, positioning LPC as a potential prognostic or diagnostic marker. Nonetheless, the connection between plasma LPC levels and stroke severity remains unclear. This study aimed to elucidate this relationship by examining plasma LPC levels in conjunction with brain LPC levels to provide a deeper understanding of the underlying mechanisms. Adult male Sprague-Dawley rats underwent transient middle cerebral artery occlusion and were randomly assigned to different groups (sham-operated, vehicle, LPC supplementation, or LPC inhibition). We measured multiple LPC species in the plasma and brain, alongside assessing sensorimotor dysfunction, cerebral perfusion, lesion volume, and markers of BBB damage, inflammation, apoptosis, and oxidative stress. Among five LPC species, plasma LPC(16:0) and LPC(18:1) showed strong correlations with sensorimotor dysfunction, lesion severity, and mechanistic biomarkers in the rat stroke model. Despite notable discrepancies between plasma and brain LPC levels, both were strongly linked to functional outcomes and mechanistic biomarkers, suggesting that LPC's prognostic value is retained extracranially. This study advances the understanding of LPC as a blood marker in ischemic stroke and highlights directions for future research to further elucidate its association with stroke severity, particularly through investigations in more clinically representative models.
Keywords: LPC(18:1); biomarker; ischemic stroke; lipid; lysophosphatidylcholine; prognostic marker; transient middle cerebral artery occlusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Kwakkel G., Veerbeek J.M., Harmeling-van der Wel B.C., van Wegen E., Kollen B.J., on behalf of the Early Prediction of functional Outcome after Stroke (EPOS) Investigators Diagnostic Accuracy of the Barthel Index for Measuring Activities of Daily Living Outcome after Ischemic Hemispheric Stroke. Stroke. 2011;42:342–346. doi: 10.1161/STROKEAHA.110.599035. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

